Question: Question 8 (8 points) Listen You have a making a solution by mixing 350.0 mL of 0.648 M HTe with exactly 245.0 mL of 0.582
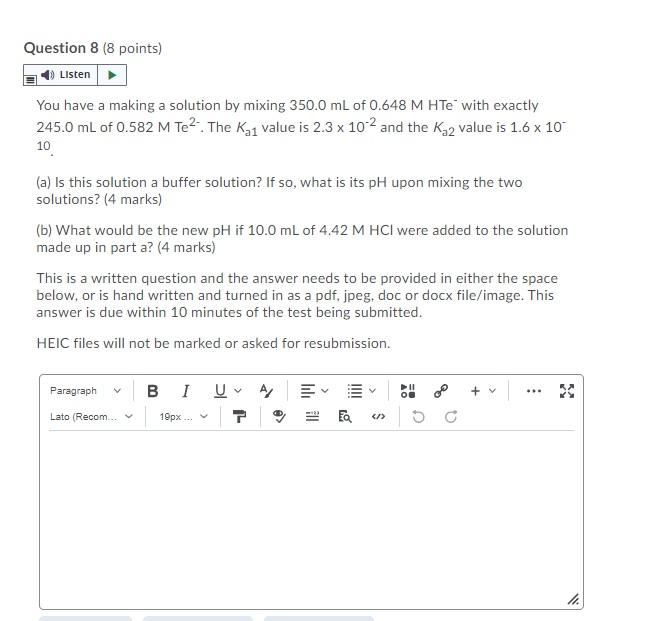
Question 8 (8 points) Listen You have a making a solution by mixing 350.0 mL of 0.648 M HTe with exactly 245.0 mL of 0.582 M Te?- The K 1 value is 2.3 x 102 and the K32 value is 1.6 x 10 10 (a) Is this solution a buffer solution? If so, what is its pH upon mixing the two solutions? (4 marks) (b) What would be the new pH if 10.0 mL of 4.42 M HCl were added to the solution made up in part a? (4 marks) This is a written question and the answer needs to be provided in either the space below, or is hand written and turned in as a pdf, jpeg, doc or docx file/image. This answer is due within 10 minutes of the test being submitted. HEIC files will not be marked or asked for resubmission. Paragraph BI A > + .. Ihill Lato (Recom... 19px... L > he
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


