Question: Given the following equation Znts) + 02e - ZnO(s) If 35.0 g of Zn is reacted with 70.0 g of O 2 - determine
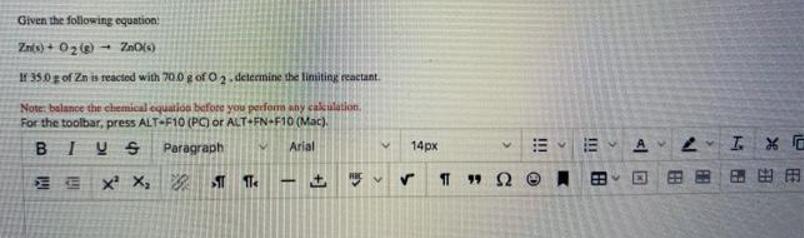
Given the following equation Znts) + 02e - ZnO(s) If 35.0 g of Zn is reacted with 70.0 g of O 2 - determine the liniting reactant. Note: balance the chemical equatioa before you perfom any cakulation. For the toolbar, press ALT-F10 (PC) or ALT+FN+F10 (Mac). BIUS Paragraph Arial 14px A E E X X, 99 2 !! !! +]
Step by Step Solution
★★★★★
3.44 Rating (151 Votes )
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock

According to the law of conservati on of mass the balanced chemical equation must have the same numb... View full answer

Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


