Question: question 9A Consider the reaction A + 2B=0 whose rate at 25C was measured using three different sets of initial concentrations as listed in the
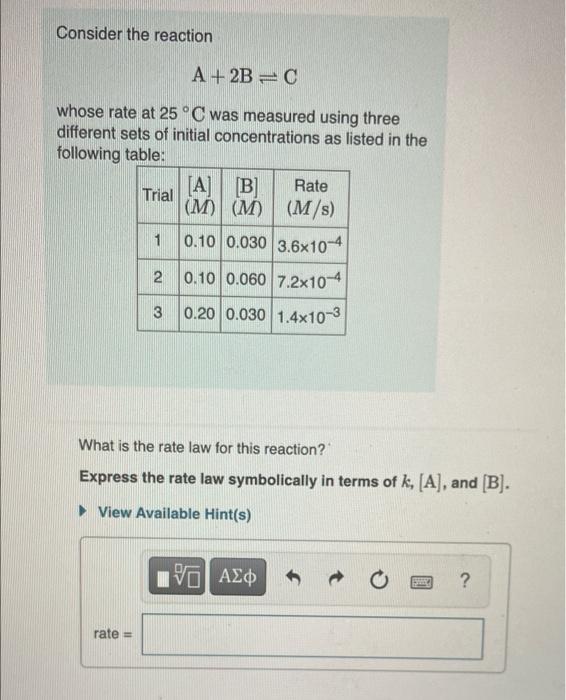
Consider the reaction A + 2B=0 whose rate at 25C was measured using three different sets of initial concentrations as listed in the following table: [A] [B] Rate Trial (M) (M) (M/s) 1 0.10 0.030 3.6x10-4 2 0.10 0.060 7.2x10-4 3 0.20 0.030 1.4x10-3 What is the rate law for this reaction? Express the rate law symbolically in terms of k, [A], and B). View Available Hint(s) IVO AE ? rate =
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


