Question: Consider the reaction: 2,0(g) o, (9) +2Na(9). Which of the following will cause a shift in the equilibrium to the right? 1. Add more
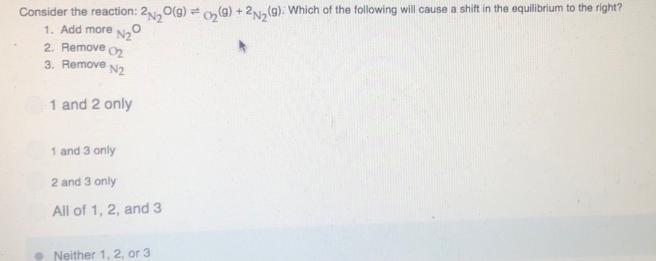
Consider the reaction: 2,0(g) o, (9) +2Na(9). Which of the following will cause a shift in the equilibrium to the right? 1. Add more Na 2. Remove o2 3. Remove N2 1 and 2 only 1 and 3 only 2 and 3 only All of 1, 2, and 3 Neither 1, 2, or 3
Step by Step Solution
3.40 Rating (156 Votes )
There are 3 Steps involved in it
Answer Option 3 1st and 3rd reaction Only the euations 1 and 3 are oxidation reduction reactions whi... View full answer

Get step-by-step solutions from verified subject matter experts


