Question: Question Completion Status: lestion 2 5 points For the strong electrolyte, the Debye-Hckel-Onsager equation is =0ac Using a conductivity meter, the specific conductance, k, values
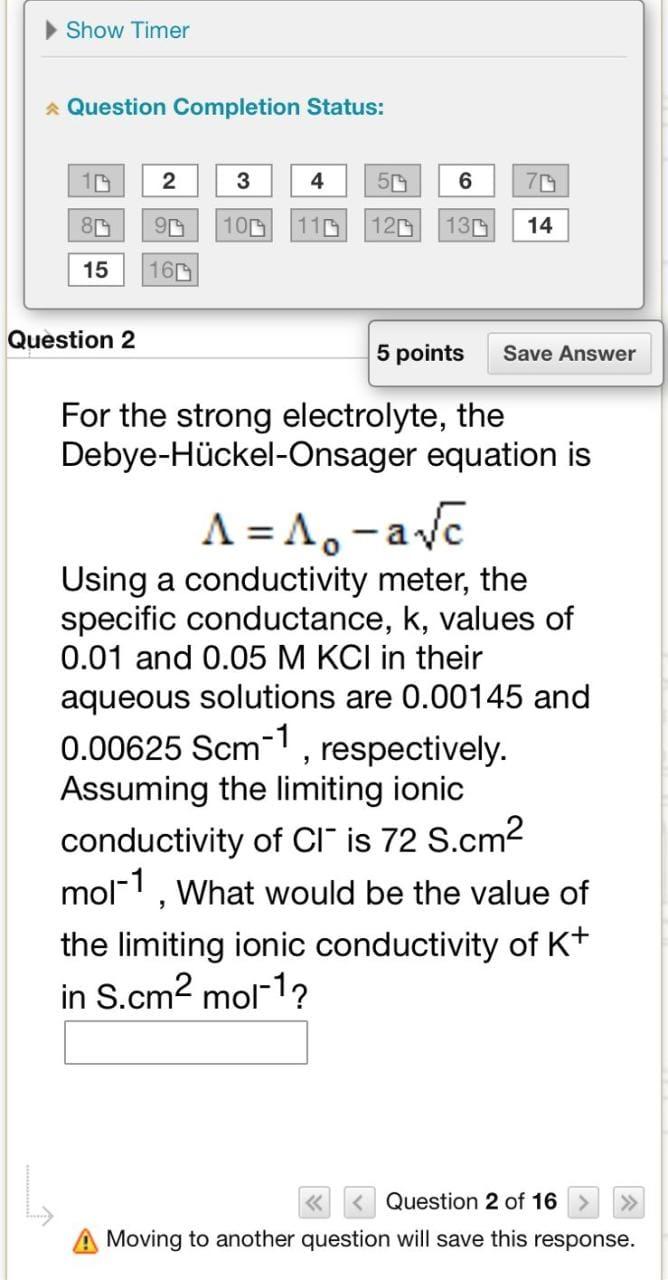
Question Completion Status: lestion 2 5 points For the strong electrolyte, the Debye-Hckel-Onsager equation is =0ac Using a conductivity meter, the specific conductance, k, values of 0.01 and 0.05MKCl in their aqueous solutions are 0.00145 and 0.00625Scm1, respectively. Assuming the limiting ionic conductivity of Clis 72 S.cm 2 mol1, What would be the value of the limiting ionic conductivity of K+ Question 2 of 16 A Moving to another question will save this response
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


