Question: For the strong electrolyte, the DebyeHckel-Onsager equation is =0ac Using a conductivity meter, the specific conductance, k, values of 0.01 and 0.05 MKCl in their
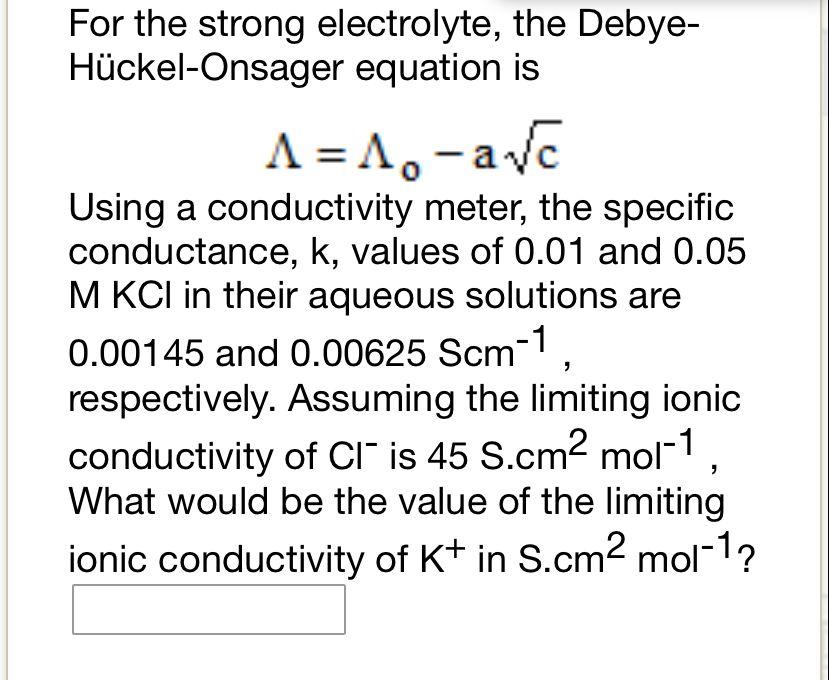
For the strong electrolyte, the DebyeHckel-Onsager equation is =0ac Using a conductivity meter, the specific conductance, k, values of 0.01 and 0.05 MKCl in their aqueous solutions are 0.00145 and 0.00625Scm1, respectively. Assuming the limiting ionic What would be the value of the limiting
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


