Question: question (e) please Q6: 16 marks An electrochemical hydrogen compressor is a device similar to a PEM fuel cell or electrolyser that operates with hydrogen
question (e) please
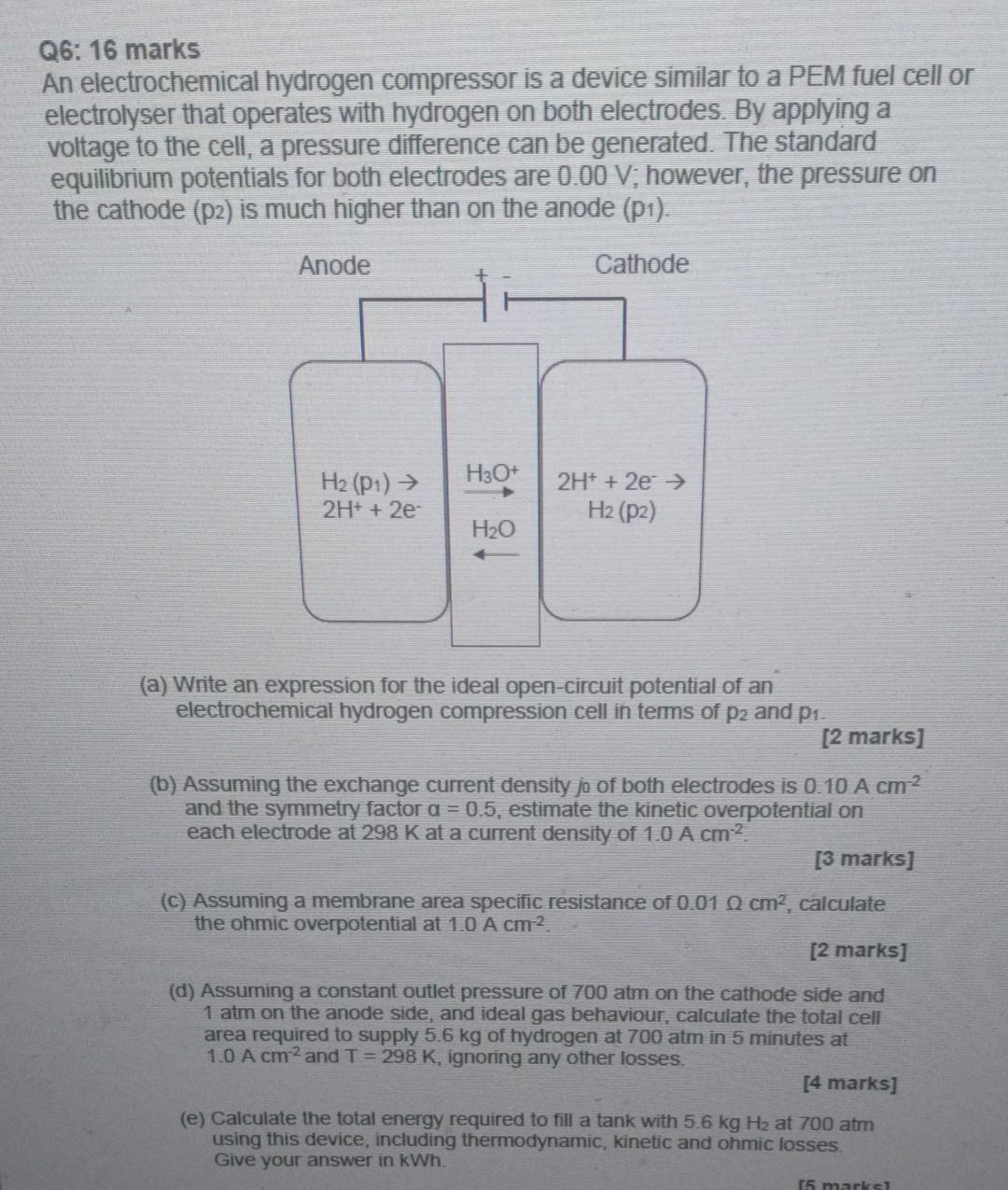
Q6: 16 marks An electrochemical hydrogen compressor is a device similar to a PEM fuel cell or electrolyser that operates with hydrogen on both electrodes. By applying a voltage to the cell, a pressure difference can be generated. The standard equilibrium potentials for both electrodes are 0.00 V; however, the pressure on the cathode (p2) is much higher than on the anode (p1). Anode Cathode H3O+ 2H+ + 2e H2 (P1) 2H+ + 2e- Hz (P2) H2O (a) Write an expression for the ideal open-circuit potential of an electrochemical hydrogen compression cell in terms of P2 and P1. [2 marks] (b) Assuming the exchange current density jo of both electrodes is 0.10 A cm2 and the symmetry factor a = 0.5, estimate the kinetic overpotential on each electrode at 298 Kat a current density of 1.0 A cm? [3 marks] (C) Assuming a membrane area specific resistance of 0.01 cm2, calculate the ohmic overpotential at 1.0 A cm2 [2 marks] (d) Assuming a constant outlet pressure of 700 atm on the cathode side and 1 atm on the anode side, and ideal gas behaviour, calculate the total cell area required to supply 5.6 kg of hydrogen at 700 atm in 5 minutes at 1.0 A cm2 and T = 298 K, ignoring any other losses. [4 marks] (e) Calculate the total energy required to fill a tank with 5.6 kg H2 at 700 atm using this device, including thermodynamic, kinetic and ohmic losses Give your answer in kWh. 15 marks
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


