Question: Question No 18 Timer: 01:15 Mark: 2 The Bohr equation for the hydrogen atom is En = -2.18 x 10-18 J2. Calculate the frequency in
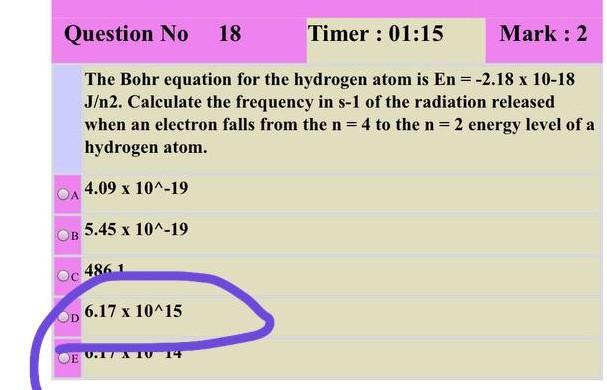
Question No 18 Timer: 01:15 Mark: 2 The Bohr equation for the hydrogen atom is En = -2.18 x 10-18 J2. Calculate the frequency in s-1 of the radiation released when an electron falls from the n = 4 to the n=2 energy level of a hydrogen atom. 4.09 x 10^-19 5.45 x 10^-19 B 4861 6.17 x 10^15 E 0.17 T 14
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


