Question: Question Six: Use the data below to construct Born-Haber cycles for lithium chloride and silver chloride, and from those work out the lattice enthalpies for
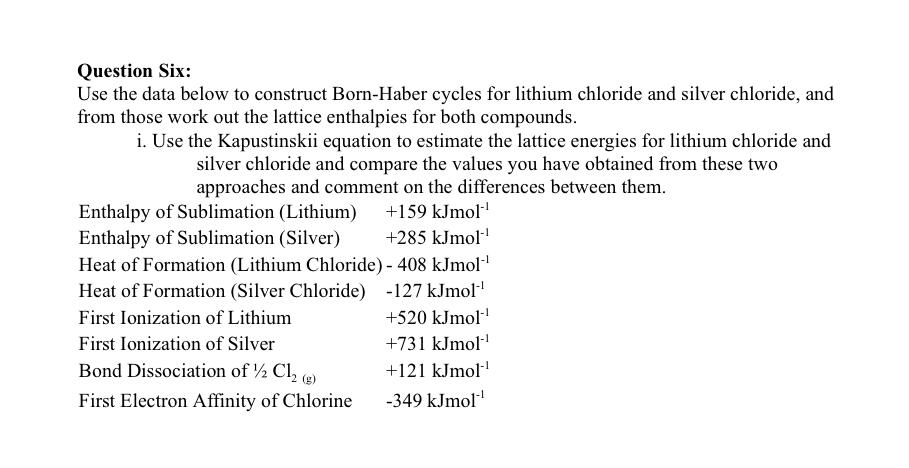
Question Six: Use the data below to construct Born-Haber cycles for lithium chloride and silver chloride, and from those work out the lattice enthalpies for both compounds. i. Use the Kapustinskii equation to estimate the lattice energies for lithium chloride and silver chloride and compare the values you have obtained from these two approaches and comment on the differences between them. Enthalpy of Sublimation (Lithium) +159 kJmol' Enthalpy of Sublimation (Silver) +285 kJmol' Heat of Formation (Lithium Chloride) - 408 kJmol! Heat of Formation (Silver Chloride) -127 kJmol"! First Ionization of Lithium +520 kJmol First Ionization of Silver +731 kJmol Bond Dissociation of 12 Ch (g) +121 kJmol' First Electron Affinity of Chlorine -349 kJmol
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


