Question: (b) (i) The following experiment is used to determine the vinyl acetate (VA) level in an ethylene vinyl acetate (EVA) commercial packaging film infrared
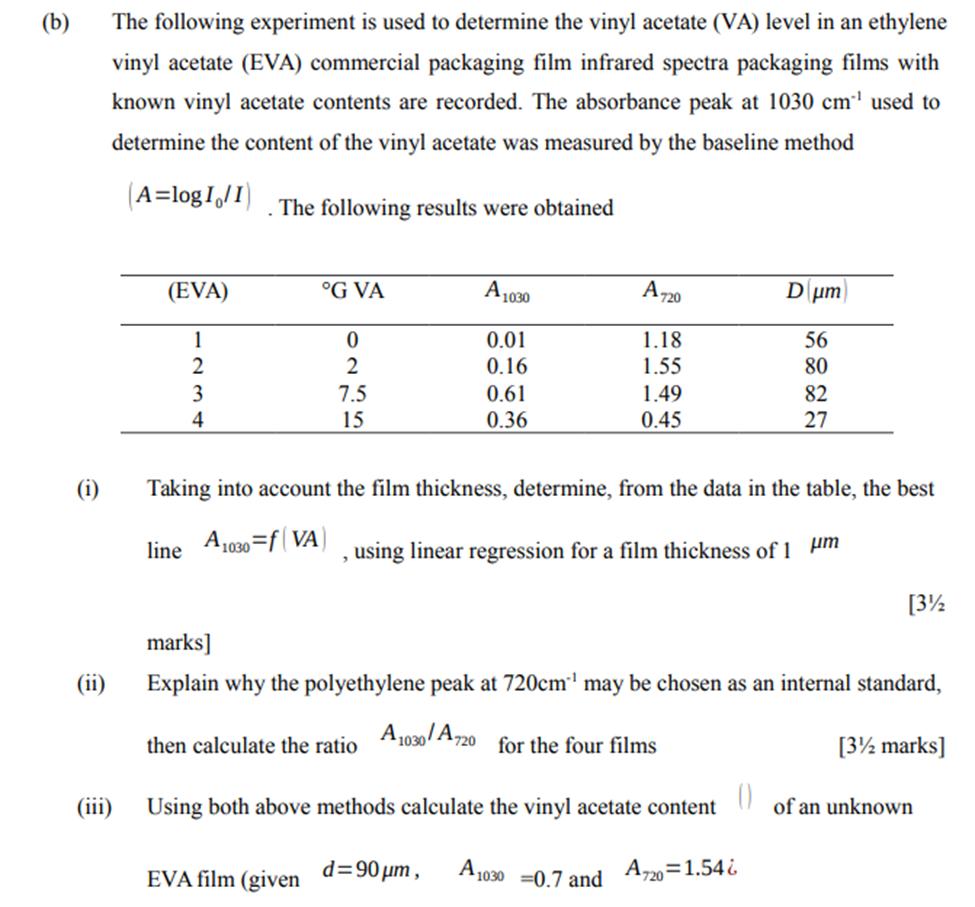
(b) (i) The following experiment is used to determine the vinyl acetate (VA) level in an ethylene vinyl acetate (EVA) commercial packaging film infrared spectra packaging films with known vinyl acetate contents are recorded. The absorbance peak at 1030 cm used to determine the content of the vinyl acetate was measured by the baseline method (A=logI/I) (EVA) 1 2 3 4 line The following results were obtained A G VA 0 2 7.5 15 EVA film (given A1030 0.01 0.16 0.61 0.36 Taking into account the film thickness, determine, from the data in the table, the best 1030=f(VA) using linear regression for a film thickness of 1 m A720 1.18 1.55 1.49 0.45 d=90 m, marks] Explain why the polyethylene peak at 720cm may be chosen as an internal standard, then calculate the ratio A1030/A720 for the four films [3 marks] Using both above methods calculate the vinyl acetate content A1030 =0.7 and A720=1.54& Dum 56 80 82 27 (0) [3% of an unknown
Step by Step Solution
3.49 Rating (162 Votes )
There are 3 Steps involved in it
i To determine the best line for A1030fVA using linear regression we need to analyze the data and find a linear relationship between the absorbance at ... View full answer

Get step-by-step solutions from verified subject matter experts


