Question: QUESTIONS TO ANSWER BEFORE THE LAB EXERCISE 1. Find the masy, in grams, of a 240ml. sample of dry air at a pressure of 750mmily

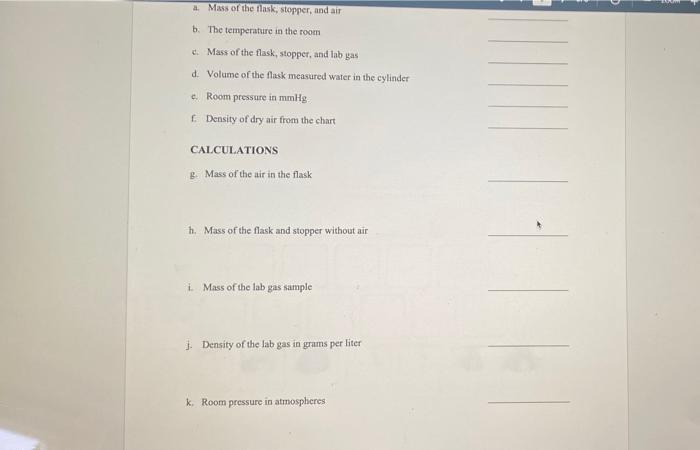
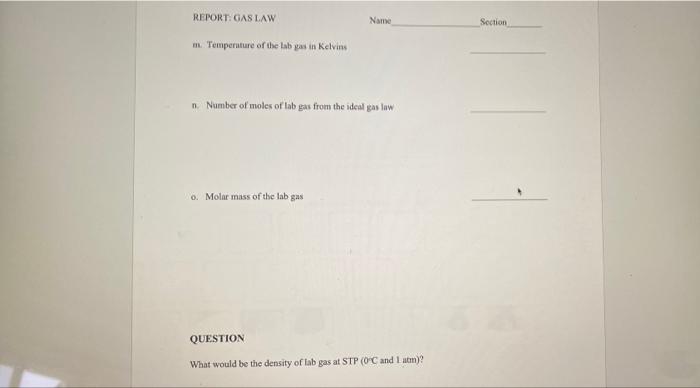
QUESTIONS TO ANSWER BEFORE THE LAB EXERCISE 1. Find the masy, in grams, of a 240ml. sample of dry air at a pressure of 750mmily and a temperature of 19c. Refer to the chart on the previous page for dry air density 2. An unknown gas sample is collected in a 25ml.contince at 25+Cand 755 mmHg How many moles of gas are present? 3. If the gas sample of question 2 was weighed and found to have a mass of 0.456 grams, what is the molar mass of the gas? 4. What was the density in grams per liter of the gas of question 2? a Mass of the flask, stopper, and air b. The temperature in the room Mass of the flask, stopper, and lab gas d. Volume of the flask measured water in the cylinder Room pressure in mmHg Density of dry air from the chart CALCULATIONS B. Mass of the air in the flask h. Mass of the flask and stopper without air i Mass of the lab gas sample 1. Density of the lab gas in grams per liter k Room pressure in atmospheres REPORT: GAS LAW Name Section m. Temperature of the lab gas in Kelvins n. Number of moles of lab gas from the ideal gas law 0. Molar mass of the lab gas QUESTION What would be the density of lab gas at STP (OPC and I am)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


