Question: Calculate the (H*| and the pH of a solution with an JOH| = 43 x 10-10 Mat 25 C. %3D 1.62 x10-13 M. pH
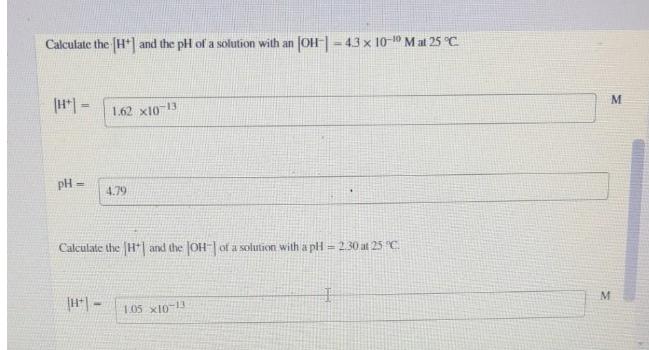
Calculate the (H*| and the pH of a solution with an JOH| = 43 x 10-10 Mat 25 C. %3D 1.62 x10-13 M. pH = 4.79 Calculate the H| and the OH of a solution with a pH = 230 at 25 C. 105 x10-1 M
Step by Step Solution
★★★★★
3.40 Rating (153 Votes )
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


