Question: Rate Law from Initial Rates ( B ) 2 C l O 2 ( a q ) + 2 O H - ( a q
Rate Law from Initial Rates B
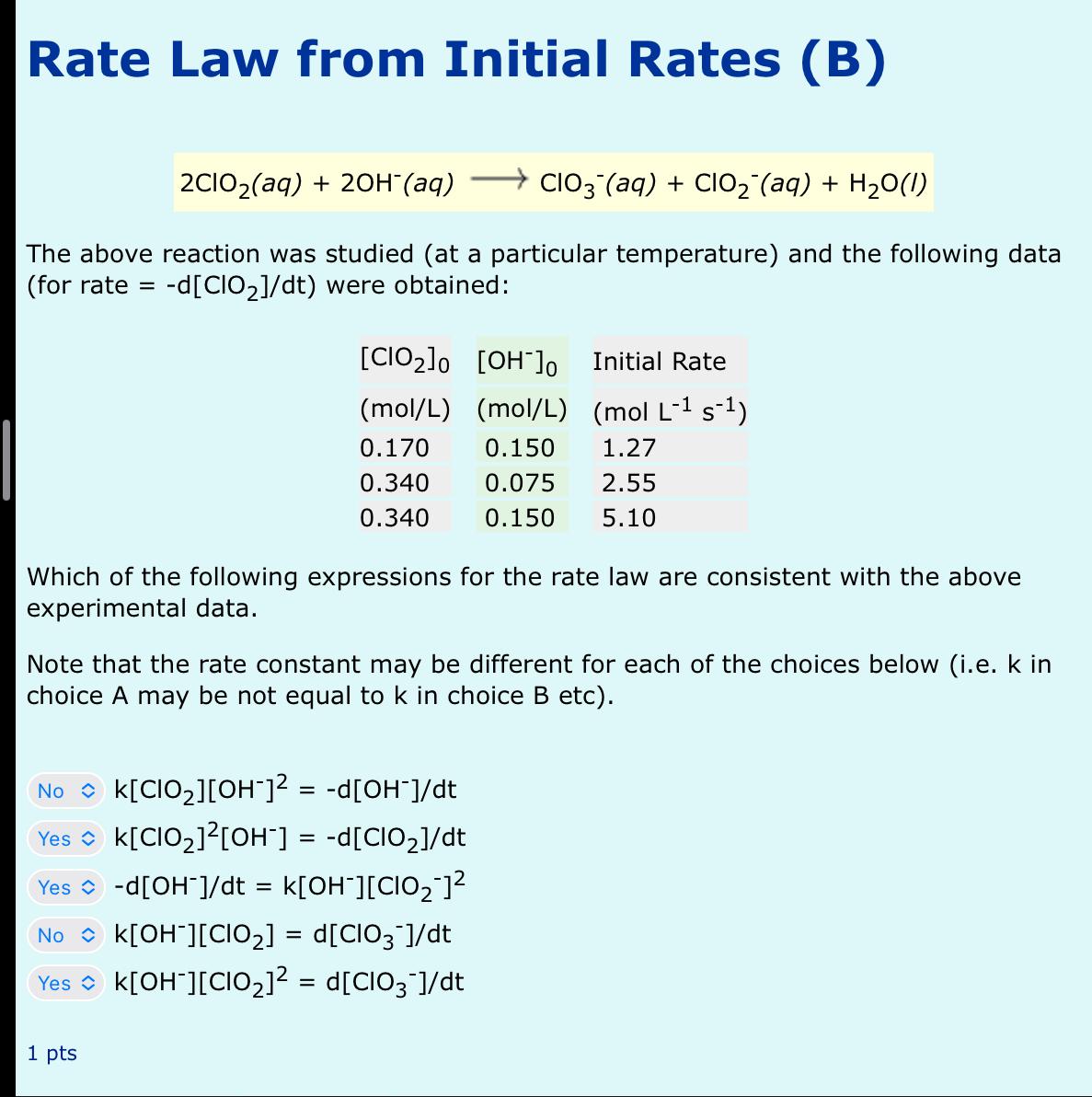
The above reaction was studied at a particular temperature and the following data for rate were obtained:
tableInitial Rate
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


