Question: REACTION A+B+C Products Using the data in the table and the rate law below. identify the reaction order with respect to each reactant. Rate =k[A]m[B]n[C]p(
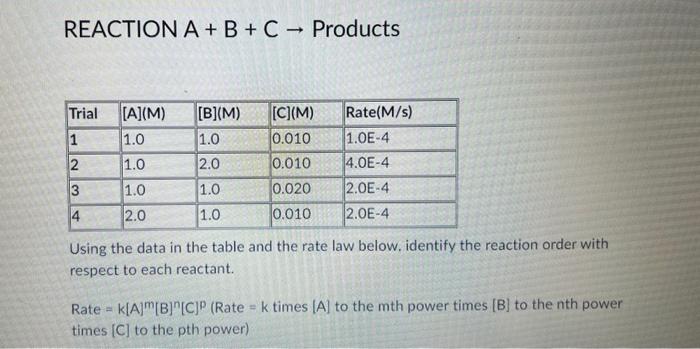
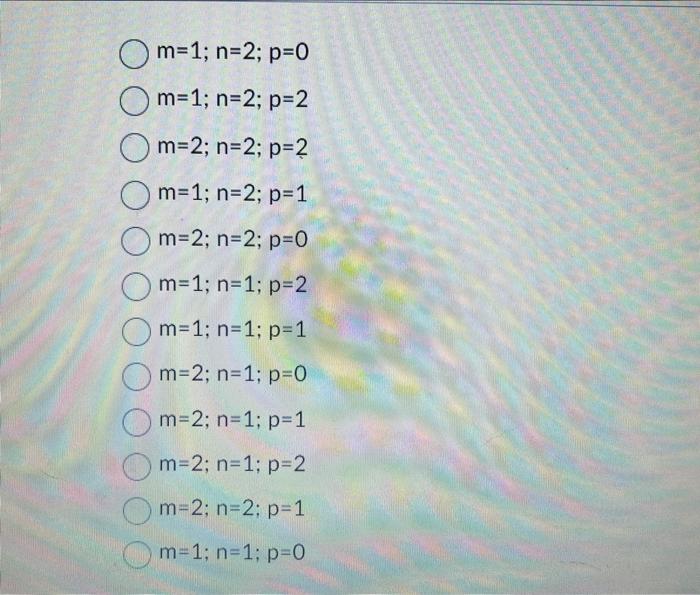
REACTION A+B+C Products Using the data in the table and the rate law below. identify the reaction order with respect to each reactant. Rate =k[A]m[B]n[C]p( Rate =k times [A] to the m th power times [B] to the nth power times [C] to the pth power) m=1;n=2;p=0 m=1;n=2;p=2 m=2;n=2;p=2 m=1;n=2;p=1 m=2;n=2;p=0 m=1;n=1;p=2 m=2;n=1;p=0 m=2;n=1;p=1 m=2;n=1;p=2 m=2;n=2;p=1 m=1;n=1;p=0
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


