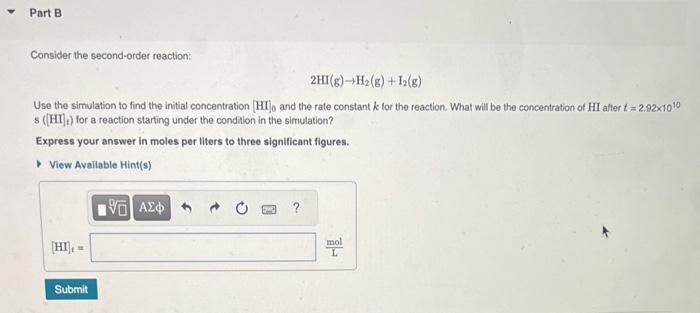
Question: Consider the second-order reaction: 2HI(g)H2(g)+I2(g) Use the simulation to find the initial concentration [HI]0 and the rate constant k tor the reaction. What will be
![initial concentration [HI]0 and the rate constant k tor the reaction. What](https://dsd5zvtm8ll6.cloudfront.net/si.experts.images/questions/2024/09/66fa28242607a_30766fa2823baeea.jpg)
![will be the concentration of HI atter t=2.921010 s([HI]t) for a reaction](https://dsd5zvtm8ll6.cloudfront.net/si.experts.images/questions/2024/09/66fa2824c5556_30866fa28245ffb1.jpg)
Consider the second-order reaction: 2HI(g)H2(g)+I2(g) Use the simulation to find the initial concentration [HI]0 and the rate constant k tor the reaction. What will be the concentration of HI atter t=2.921010 s([HI]t) for a reaction starting under the condition in the simulation? Express your answer in moles per liters to three significant figures. Characteristics of second-order reactions For a second-order reaction, [A] products, the rate of the reaction is given as rate =k[A]2, where k is the rate constant and [A] is the concentration of reactant A. The integrated rate law for second-order reactions is [A]t1=kt+[A]01, where [A]t is the concentration of reactant A at time t,k is the rate constant, and [A]0 is the initial concentration of reactant A. This equation is of the type y=mx+b. Therelore, the plot of [A]t1 versus time is always a straight line with a siope k and a y intercept [A]01. The piot of [A] versus t is linear for the zero-order reaction, the plot of ln[A] versus t is linear for the first-order reaction, and the plot of [A1 versus t is linear for the second-order reaction. [A] represents the concentration of the reactant A. The linearity of each graph can be used to identify the order of a reaction. Part A There are six different reactions you can access in the simulation using the drop-down menu. Which of the following are second-order reactions? Check all that apply. Viow Avallable Hint(s) C2H62CH32N2O2N2+O22NO22NO+O22HIH2+I22N2O54NO2+O2 (P) Pearson
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


