Question: REACTION During decomposition, one compound splits apart into two or more substances. DESCRIPTION: These substances can be elements or simpler compounds. REACTION FORMAT: AB -
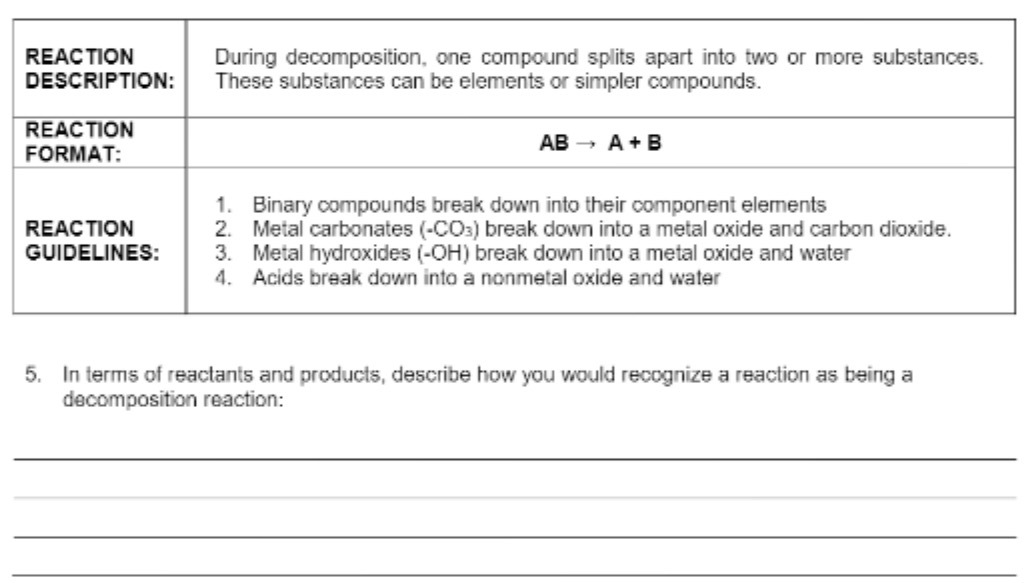
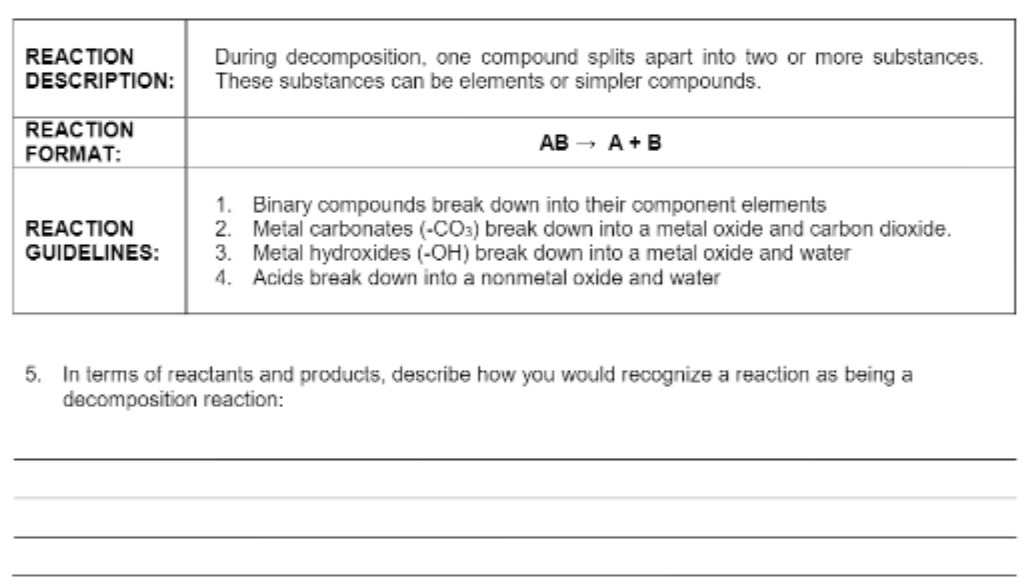
REACTION During decomposition, one compound splits apart into two or more substances. DESCRIPTION: These substances can be elements or simpler compounds. REACTION FORMAT: AB - A+ B 1. Binary compounds break down into their component elements REACTION 2. Metal carbonates (-CO3) break down into a metal oxide and carbon dioxide. GUIDELINES: 3. Metal hydroxides (-OH) break down into a metal oxide and water 4. Acids break down into a nonmetal oxide and water 5. In terms of reactants and products, describe how you would recognize a reaction as being a decomposition reaction
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


