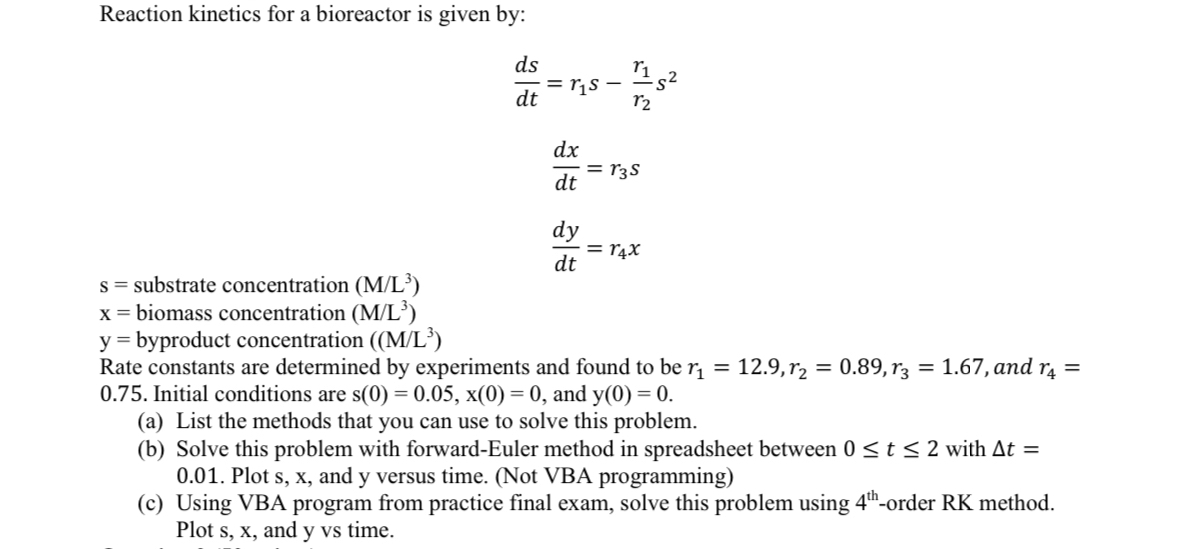
Question: Reaction kinetics for a bioreactor is given by: d s d t = r 1 s - r 1 r 2 s 2 d x
Reaction kinetics for a bioreactor is given by:
substrate concentration
biomass concentration
byproduct concentration
Rate constants are determined by experiments and found to be and Initial conditions are and
a List the methods that you can use to solve this problem.
b Solve this problem with forwardEuler method in spreadsheet between with Plot and y versus time. Not VBA programming
c Using VBA program from practice final exam, solve this problem using order RK method. Plot and y vs time.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


