Question: Really need help with this question the specific internal energies at 0 C, 150 C, & 17.5 C are (-460.8, 3913.3, & -0.0046 (J/mol)) The
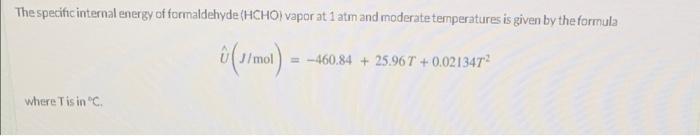
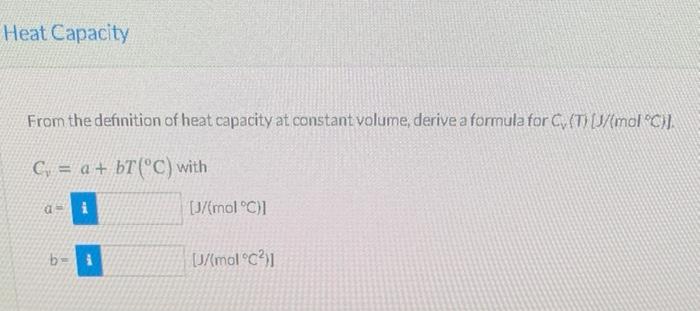
The specific internal energy of formaldehyde (HCHO) vapor at 1 atm and moderate temperatures is given by the formula 6(1/mol) =-460.84 + 25.967 +0.021347 where Tisin Heat Capacity From the definition of heat capacity at constant volume, derive a formula for C (T) (1/(mol "C)]. C = a + bT(C) with a i [J/(mol01 b- [/(molC?)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


