Question: Refer to the temperature versus time graph (Figure 2) when answering the questions in Parts C through F. A system consists of 250 g of
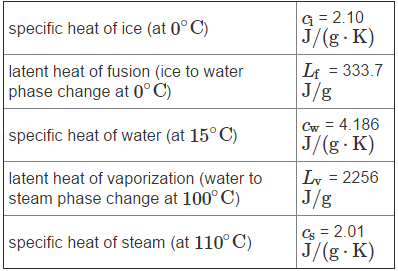
Refer to the temperature versus time graph (Figure 2) when answering the questions in Parts C through F. A system consists of 250 g of water. The system, originally at T A = 21.0 ?C, is placed in a freezer, where energy is removed from it in the form of heat at a constant rate. The figure shows how the temperature of the system takes t 1 = 10 min = 600 s to drop to 0? C, after which the water freezes. Once the freezing is complete, the temperature of the resulting ice continues to drop, reaching temperature T B after an hour. The following specific heat and latent heat values for water may be helpful.
How much energy must be transferred out of the system as heat Q to lower its temperature to 0?C?
ANSWER Q = 2.20 x 10^4
Cooling Power of P is 36.6 W
At what time t 2 will the water be completely frozen so the temperature can begin to fall below 0?C?
(seconds)
specific heat of ice (at 0C) latent heat of fusion (ice to water phase change at 0C) specific heat of water (at 15C) latent heat of vaporization (water to steam phase change at 100 C) specific heat of steam (at 110 C) G = 2.10 J/(g.K) Lf = 333.7 J/g Cw = 4.186 J/(g.K) Lv = 2256 J/g Cs = 2.01 J/(g.K)
Step by Step Solution
3.40 Rating (166 Votes )
There are 3 Steps involved in it
To find the time t2 when the water is completely frozen follow these steps Step 1 Calculate the he... View full answer

Get step-by-step solutions from verified subject matter experts


