Question: A patient is suspected of having low stomach acid, a condition known as hypochloridia. To determine whether the patient has this condition, her doctors
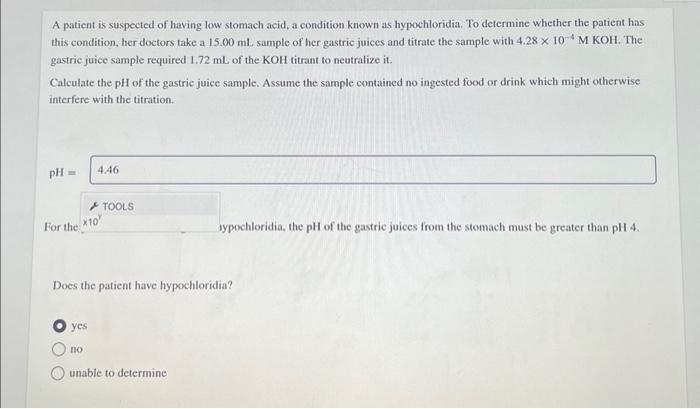
A patient is suspected of having low stomach acid, a condition known as hypochloridia. To determine whether the patient has this condition, her doctors take a 15.00 ml. sample of her gastric juices and titrate the sample with 4.28 x 10-4 M KOH. The gastric juice sample required 1.72 mL of the KOH titrant to neutralize it. Calculate the pH of the gastric juice sample. Assume the sample contained no ingested food or drink which might otherwise interfere with the titration. pH = For the 4.46 *10* yes TOOLS no Does the patient have hypochloridia? sypochloridia, the pH of the gastric juices from the stomach must be greater than pH 4. unable to determine
Step by Step Solution
3.44 Rating (157 Votes )
There are 3 Steps involved in it
Answer To calculate the pH of the gastric juice sample we can use the formula for pH pHlogHpHlogH Fi... View full answer

Get step-by-step solutions from verified subject matter experts


