Question: Remember to draw a schematic and write any assumptions for each problem. Show all work and include units. 1. One kilogram of an ideal
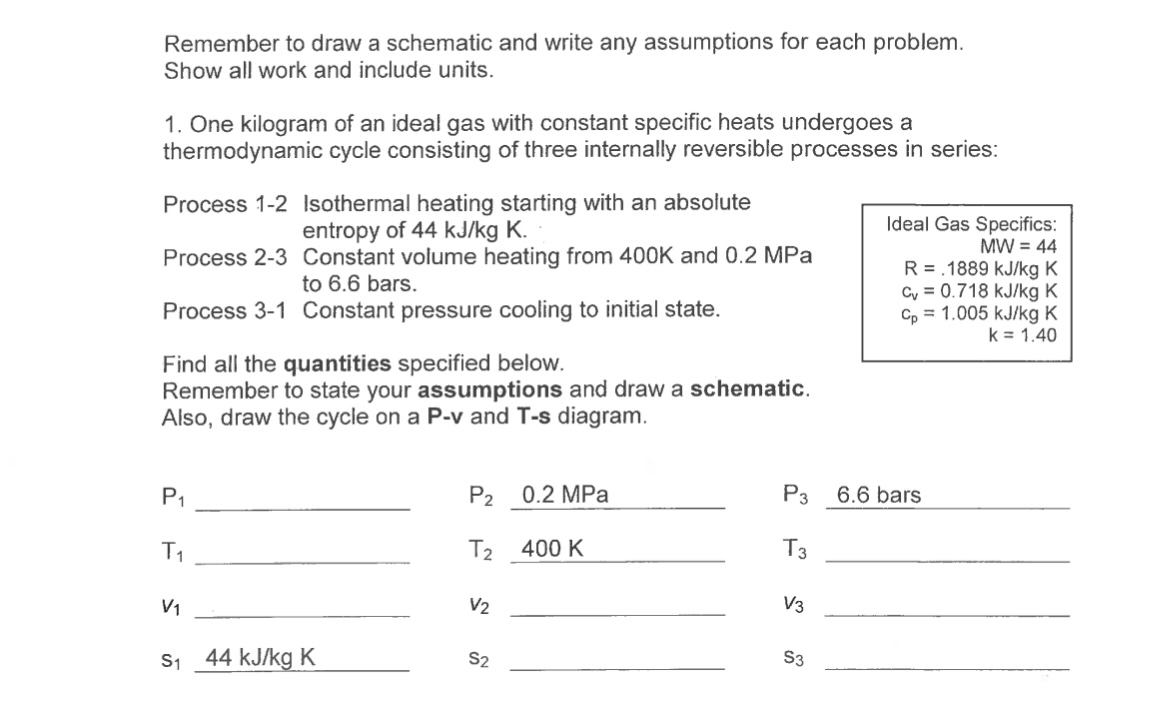
Remember to draw a schematic and write any assumptions for each problem. Show all work and include units. 1. One kilogram of an ideal gas with constant specific heats undergoes a thermodynamic cycle consisting of three internally reversible processes in series: Process 1-2 Isothermal heating starting with an absolute entropy of 44 kJ/kg K. Process 2-3 Constant volume heating from 400K and 0.2 MPa to 6.6 bars. Process 3-1 Constant pressure cooling to initial state. Find all the quantities specified below. Remember to state your assumptions and draw a schematic. Also, draw the cycle on a P-v and T-s diagram. Ideal Gas Specifics: MW=44 R = .1889 kJ/kg K C = 0.718 kJ/kg K Cp = 1.005 kJ/kg K k = 1.40 P1 T = P2 0.2 MPa P3 6.6 bars T 400 K T3 V3 V2 S3 V1 S 44 kJ/kg K S2
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


