Question: Report - Distribution Coefficient (1pt) Distribution Coefficient How will you collect data for this experiment? virtually Initial solution and extracting solvent data Concentration of benzoic

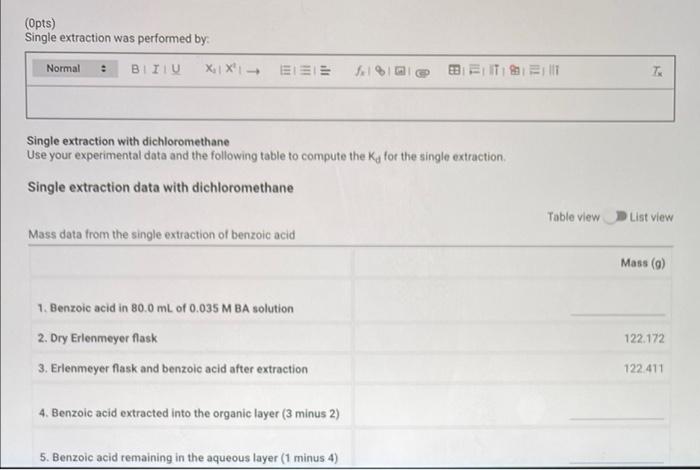
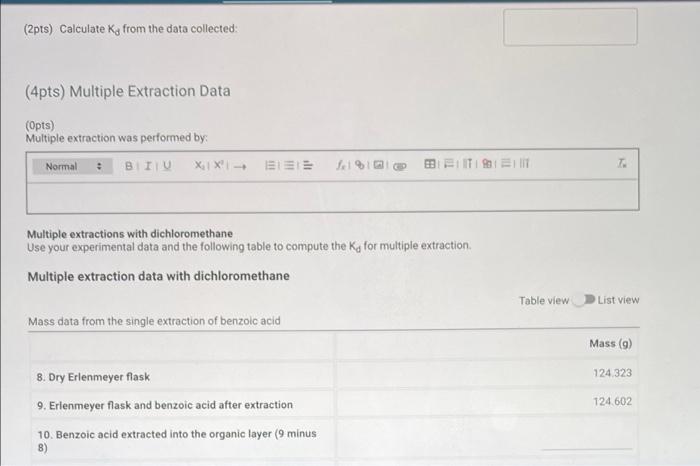

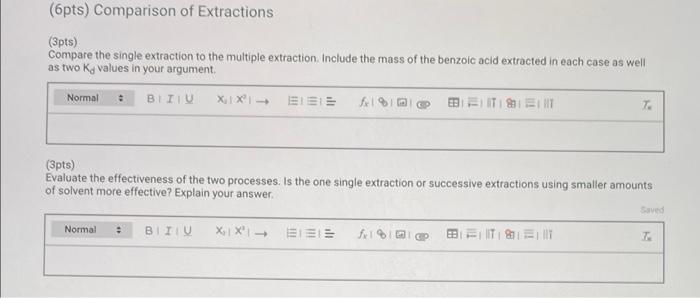
Report - Distribution Coefficient (1pt) Distribution Coefficient How will you collect data for this experiment? virtually Initial solution and extracting solvent data Concentration of benzoic acid solution (M). Volume of benzoic acid solution (ml.): Total volume of dichloromethane used during extraction (ml): 0.035 80 45.0 122.172 122.411 Single Extraction Data Mass of dry Erlenmeyer flask (9) Mass of Erlenmeyer flask and benzoic acid after extraction (9) Multiple Extraction Data Mass of dry Erlenmeyer flask (9) Mass of Erlenmeyer flask and benzoic acid after extraction (9) 124.323 124.602 (Opts) Single extraction was performed by: Normal XiX'! - EEE 1% Bill Single extraction with dichloromethane Use your experimental data and the following table to compute the Ka for the single extraction Single extraction data with dichloromethane Table view List view Mass data from the single extraction of benzoic acid Mass (6) 1. Benzoic acid in 80.0 mL of 0.035 M BA solution 2. Dry Erlenmeyer flask 3. Erlenmeyer flask and benzoic acid after extraction 122172 122.411 4. Benzoic acid extracted into the organic layer (3 minus 2) 5. Benzoic acid remaining in the aqueous layer (1 minus 4) (2pts) Calculate Ka from the data collected: (4pts) Multiple Extraction Data (Opts) Multiple extraction was performed by: Normal. BTU XIX- ELEE fel a BB ELITIS ET T: Multiple extractions with dichloromethane Use your experimental data and the following table to compute the Ka for multiple extraction Multiple extraction data with dichloromethane Table view List View Mass data from the single extraction of benzoic acid Mass (9) 124 323 124.602 8. Dry Erlenmeyer flask 9. Erlenmeyer flask and benzoic acid after extraction 10. Benzoic acid extracted into the organic layer (9 minus 8) Multiple extraction data with dichloromethane Table view List view Mass data from the single extraction of benzoic acid Mass (9) 124.323 124,602 8. Dry Erlenmeyer flask 9. Erlenmeyer flask and benzoic acid after extraction 10. Benzoic acid extracted into the organic layer (9 minus 8) 11. Benzoic acid remaining in the aqueous layer (1 minus 10) (2pts) Calculate Ky from the data collected: (6pts) Comparison of Extractions (3pts) Compare the single extraction to the multiple extraction. Include the mass of the benzoic acid extracted in each case as well as two Ky values in your argument, Normal BIU X1 X- EEE feln = TEM 7 (3pts) Evaluate the effectiveness of the two processes. Is the one single extraction or successive extractions using smaller amounts of solvent more effective? Explain your answer. Saved Normal X1 X SIE | || =1
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


