Question: Report the rate as a positive quantity. 5 Work Problem For the reaction 2H2(g)+O2(g)2H2OKg) it was found that at a given temperature and after a
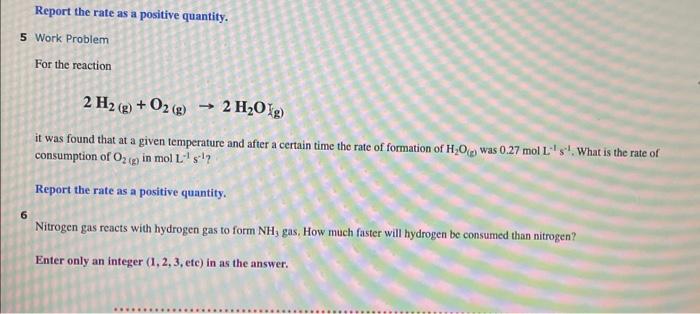
Report the rate as a positive quantity. 5 Work Problem For the reaction 2H2(g)+O2(g)2H2OKg) it was found that at a given temperature and after a certain time the rate of formation of H2O(e) was 0.27molL1s1. What is the rate of consumption of O2(g) in molL1s1 ? Report the rate as a positive quantity. 6 Nitrogen gas reacts with hydrogen gas to form NH3 gas. How much faster will hydrogen be consumed than nitrogen? Enter only an integer (1,2,3, ete) in as the
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


