Question: Reposting because expert response provided earlier was incorrect. Answer is not 0.20 and 0.14. Reaction X>Y is a single step reaction, with a deltaG (
Reposting because expert response provided earlier was incorrect. Answer is not 0.20 and 0.14. 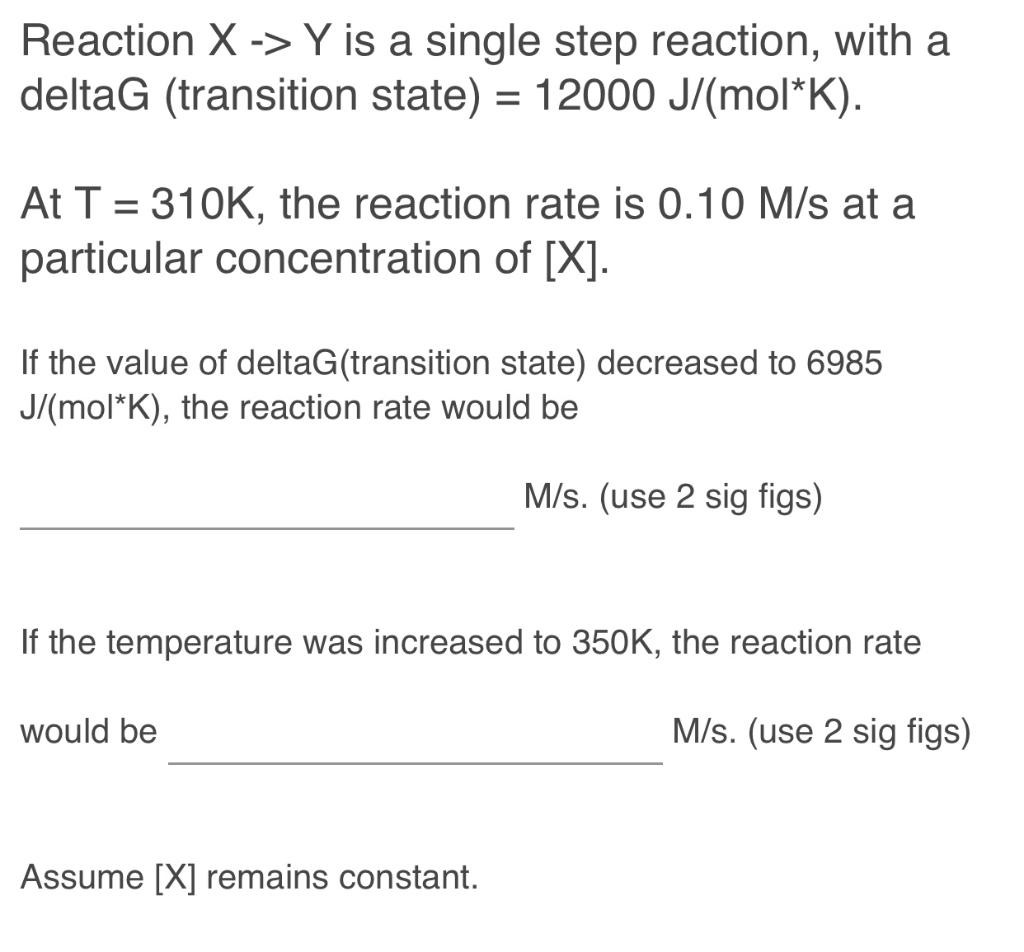
Reaction X>Y is a single step reaction, with a deltaG ( transition state )=12000J/(molK). At T=310K, the reaction rate is 0.10M/s at a particular concentration of [X]. If the value of deltaG(transition state) decreased to 6985 J/(molK), the reaction rate would be M/s. (use 2 sig figs) If the temperature was increased to 350K, the reaction rate would be M/s. (use 2 sig figs) Assume [X] remains constant
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


