Question: Required Information The ( P V ) dlagram shown is for a heat engine that uses 1 . 1 4 0 mol of
Required Information
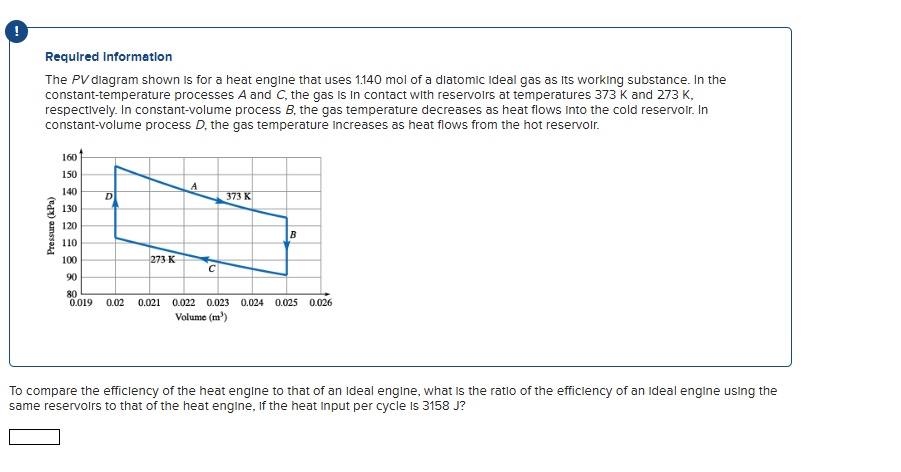
The P V dlagram shown is for a heat engine that uses mol of a diatomic ideal gas as its working substance. In the constanttemperature processes A and C the gas is in contact with reservoirs at temperatures K and K respectively. In constantvolume process B the gas temperature decreases as heat flows into the cold reservoir. In constantvolume process D the gas temperature increases as heat flows from the hot reservolr.
To compare the efficlency of the heat engine to that of an Ideal engine, what is the ratio of the efficlency of an ideal engine using the same reservoirs to that of the heat engine, If the heat input per cycle is J
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


