Question: reservoir with a solid layer of salt and then adding fresh water to a depth L=1.0m at time t=0. Diffusion of the salt into the
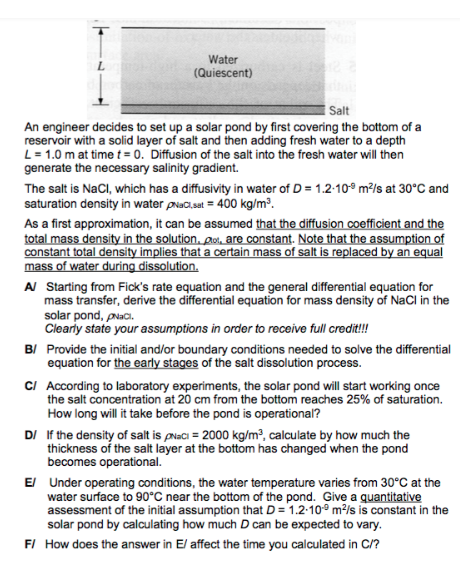
reservoir with a solid layer of salt and then adding fresh water to a depth L=1.0m at time t=0. Diffusion of the salt into the fresh water will then generate the necessary salinity gradient. The salt is NaCl, which has a diffusivity in water of D=1.2109m2/s at 30C and saturation density in water Nall,sat =400kg/m3. As a first approximation, it can be assumed that the diffusion coefficient and the total mass density in the solution, 01 are constant. Note that the assumption of constant total density implies that a certain mass of salt is replaced by an equal mass of water during dissolution. A Starting from Fick's rate equation and the general differential equation for mass transfer, derive the differential equation for mass density of NaCl in the solar pond, Nac. Clearly state your assumptions in order to receive full credit!!! B/ Provide the initial and/or boundary conditions needed to solve the differential equation for the early stages of the salt dissolution process. Cl According to laboratory experiments, the solar pond will start working once the salt concentration at 20cm from the bottom reaches 25% of saturation. How long will it take before the pond is operational? DI If the density of salt is NaCl=2000kg/m3, calculate by how much the thickness of the salt layer at the bottom has changed when the pond becomes operational. E/ Under operating conditions, the water temperature varies from 30C at the water surface to 90C near the bottom of the pond. Give a quantitative assessment of the initial assumption that D=1.2109m2/s is constant in the solar pond by calculating how much D can be expected to vary. F/ How does the answer in E/ affect the time you calculated in C/
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


