Question: Results Data for Reaction Rate vs. Concentration Graphs Note: Make sure to scroll all the way to the right so that you can complete all
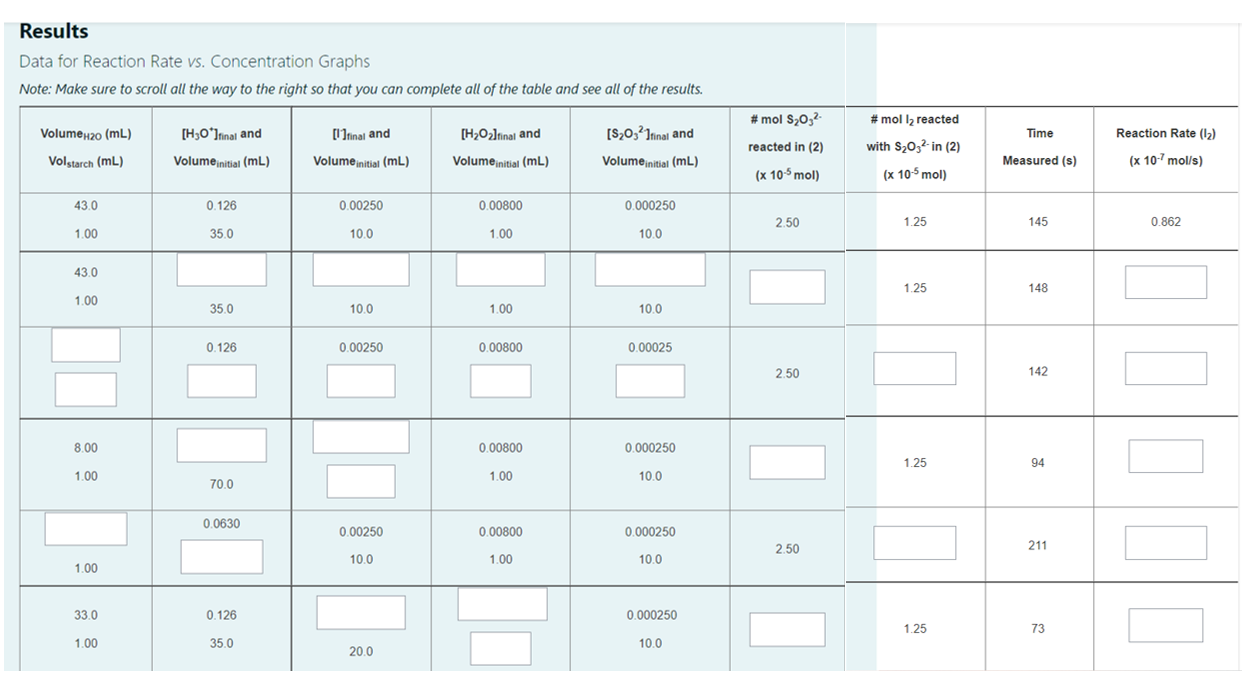
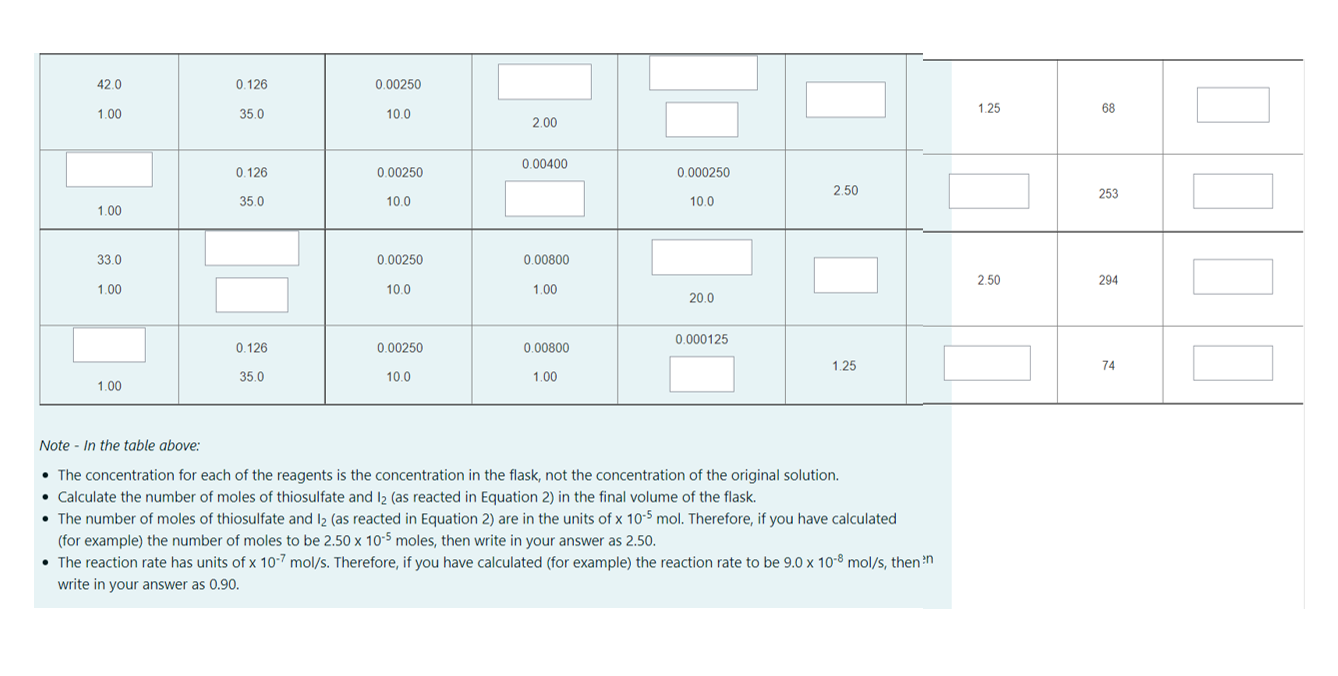
Results Data for Reaction Rate vs. Concentration Graphs Note: Make sure to scroll all the way to the right so that you can complete all of the table and see all of the results. #mol la reacted Volume 2o (mL) [H3O*Itinal and [Itinal and [H2O2Irinal and Time Reaction Rate (12) [S2032 Irinal and Volume initial (mL) #mol S2032 reacted in (2) (x 10-5 mol) ( with $20,2-in (2) Volstarch (mL) Volume initial (mL) Volume initial (mL) Volume initial (mL) Measured (s) (x 10-7 mol/s) (x 10-5 mol) 43.0 0.126 0.00250 0.00800 0.000250 2.50 1.25 145 0.862 1.00 35.0 10.0 1.00 10.0 43.0 1.25 148 1.00 35.0 10.0 1.00 10.0 0.126 0.00250 0.00800 0.00025 2.50 142 8.00 0.00800 0.000250 1.25 94 1.00 1.00 10.0 70.0 0.0630 0.00250 0.00800 0.000250 2.50 211 10.0 1.00 10.0 1.00 33.0 0.126 0.000250 1.25 73 1.00 35.0 10.0 20.0 42.0 0.126 0.00250 1.00 35.0 10.0 1.25 I 68 2.00 0.126 0.00400 0.00250 0.000250 2.50 35.0 10.0 10.0 253 1.00 33.0 0.00250 0.00800 2.50 294 1.00 10.0 1.00 20.0 0.000125 0.126 0.00250 0.00800 1.25 74 35.0 10.0 1.00 1.00 Note - In the table above: The concentration for each of the reagents is the concentration in the flask, not the concentration of the original solution. Calculate the number of moles of thiosulfate and 12 (as reacted in Equation 2) in the final volume of the flask. The number of moles of thiosulfate and 12 (as reacted in Equation 2) are in the units of x 10-5 mol. Therefore, if you have calculated (for example, the number of moles to be 2.50 x 10-5 moles, then write in your answer as 2.50. The reaction rate has units of x 10-7 mol/s. Therefore, if you have calculated (for example) the reaction rate to be 9.0 x 10-8 mol/s, then in write in your answer as 0.90
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


