Question: reversible heating and work: Tds = we have from the 1st and 2nd Laws of Thermodynamics for de + pd (1) = dh -
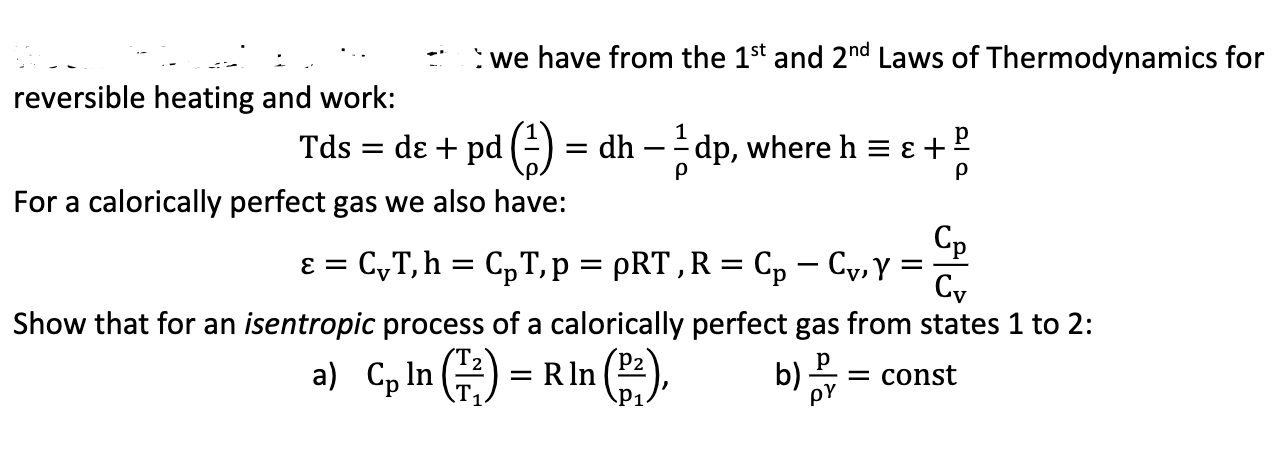
reversible heating and work: Tds = we have from the 1st and 2nd Laws of Thermodynamics for de + pd (1) = dh - 1 dp, where h = + For a calorically perfect gas we also have: = CT, h = CpT, p = pRT, R = Cp Cv, Y = Cv Show that for an isentropic process of a calorically perfect gas from states 1 to 2: a) Cp In (2) = R In P1 b) = const
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


