Question: Review TOPIC Use the References to access important values if needed for this question. The following Lewis diagram represents the valence electron configuration of a
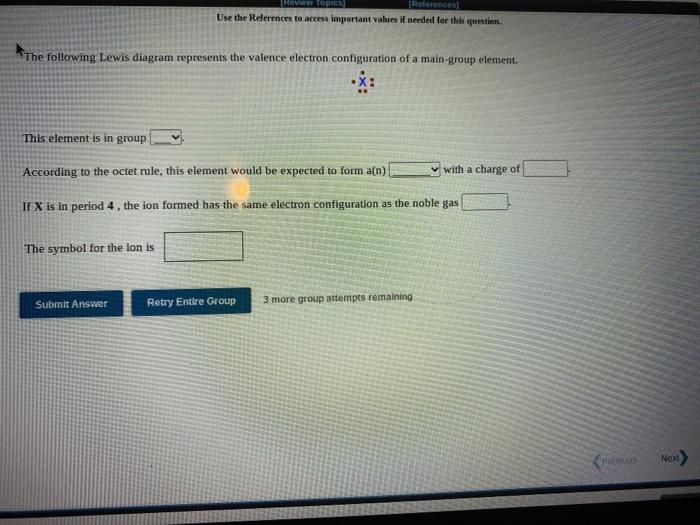
Review TOPIC Use the References to access important values if needed for this question. The following Lewis diagram represents the valence electron configuration of a main-group element X: This element is in group According to the octet rule, this element would be expected to form a(n) with a charge of If X is in period 4, the ion formed has the same electron configuration as the noble gas The symbol for the lon is Submit Answer Retry Entire Group 3 more group attempts remaining Poovus Next
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


