Question: Use the References to access important values if needed for this question. 2:X+Y:X:X: The Lewis representation above depicts a reaction between a halogen (blue) and
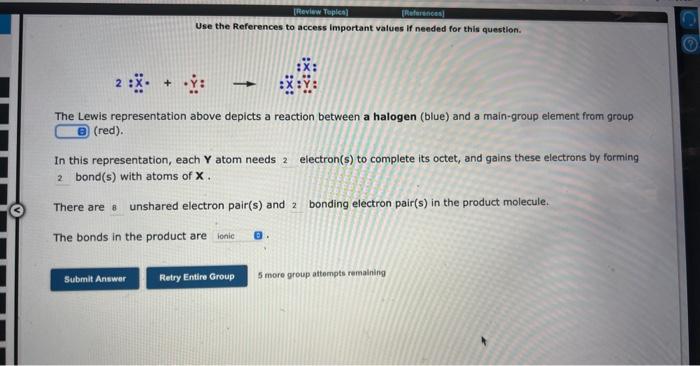
Use the References to access important values if needed for this question. 2:X+Y:X:X: The Lewis representation above depicts a reaction between a halogen (blue) and a main-group element from group (red). In this representation, each Y atom needs electron(s) to complete its octet, and gains these electrons by forming bond(s) with atoms of X. There are unshared electron pair(s) and bonding electron pair(s) in the product molecule. The bonds in the product are 5 more group attempts remaining
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


