Question: [REVIEW Topics (References Use the References to access important values if needed for this question The radius of a palladium atom is 137 pm. How
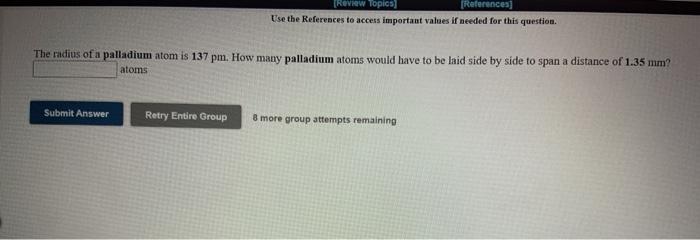
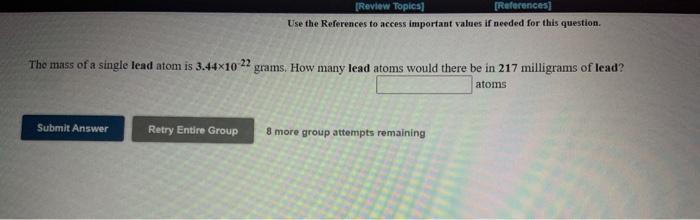
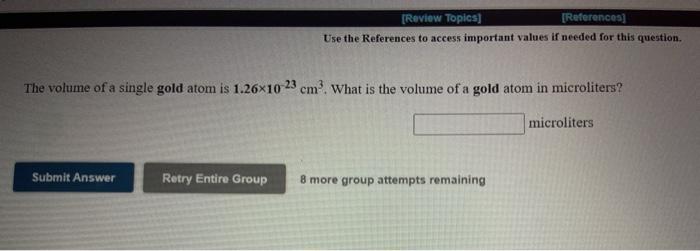
[REVIEW Topics (References Use the References to access important values if needed for this question The radius of a palladium atom is 137 pm. How many palladium atoms would have to be laid side by side to span a distance of 1.35 mm? atoms Submit Answer Retry Entire Group 8 more group attempts remaining [Review Topics] [References] Use the References to access important values if needed for this question. The mass of a single lead atom is 3.44x10 22 grams. How many lead atoms would there be in 217 milligrams of lead? atoms Submit Answer Retry Entire Group 8 more group attempts remaining [Review Topics) [References] Use the References to access important values if needed for this question. The volume of a single gold atom is 1.26x10 23 cm. What is the volume of a gold atom in microliters? microliters Submit Answer Retry Entire Group 8 more group attempts remaining
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


