Question: Provided steps would be much appreciated! Solid nickel oxide has the same kind of crystal structure as NaCl which is pictured below: CI Na If
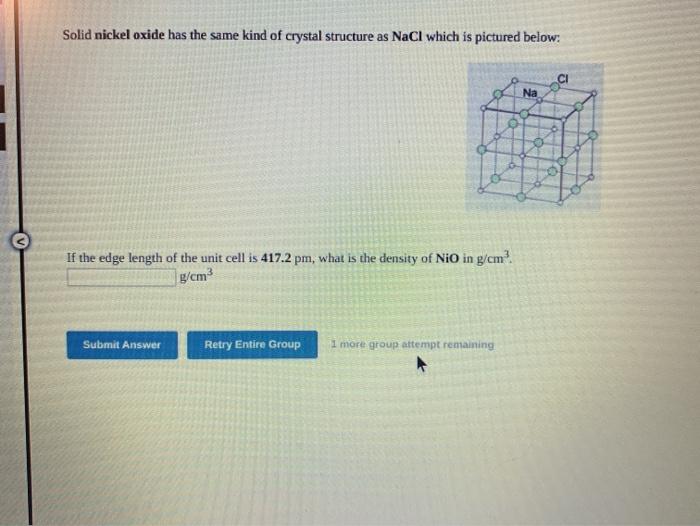
Solid nickel oxide has the same kind of crystal structure as NaCl which is pictured below: CI Na If the edge length of the unit cell is 417.2 pm, what is the density of NiO in g/cm? g/cm Submit Answer Retry Entire Group I more group attempt remaining Use the References to access important values if needed for this question. Metallic rhodium crystallizes in a face-centered cubic lattice, with one Rh atom per lattice point. If the metallic radius of Rh is 124 mm, what is the volume of the unit cell in pm and in cm Py? Submit Answer Retry Enaire Group 7 more groups remaining The substance alpha manganese is found to crystallize in a cuble lattice, with an edge lenghof 829.4 pm. If the density of solid alpha manganese is 7.521 cm how many Mn atoms are there per unit cel? Your answer should be an integer atoms Submit Answer Retry Entire Group 2 grupos mining
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


