Question: Rice husks with a certain composition (mass fraction) (see table) are burned with excess air. The heat from the combustion is used for drying grain.
Rice husks with a certain composition (mass fraction) (see table) are burned with excess air. The heat from the combustion is used for drying grain. Some of the heat is lost in the flue gas at a certain temperature. a. Draw a block diagram and complete it with the known variables. b. Calculate the specific air requirement (AF/R) (kg/kg rice husk). C. Calculate the flue gas composition and flue gas flow rate at various % excess air. d. Calculate flue gas heat loss (Qloss) and usable heat (Qmant). e. Calculate the % heat loss of flue gas against the heat carried by the fuel with 20% excess air.
Simulate and present the calculation results in the form of tables and graphs.
Heat capacity data (Cp): CO, = 37 J/mol-K, H,O = 34 J/mol:K, 02 = 29 J/mol -K, N, = 29 J/mol-K, Cp ash = 1.55 J/g.C, Tref = 25 C
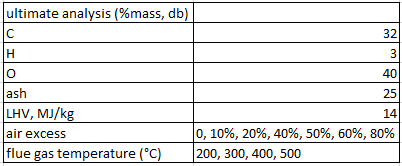
ultimate analysis (%mass, db) | 32 UIO H 3 40 25 ash LHV, MJ/kg air excess flue gas temperature (C) 14 0, 10%, 20%, 40%, 50%, 60%, 80% 200, 300, 400, 500 ultimate analysis (%mass, db) | 32 UIO H 3 40 25 ash LHV, MJ/kg air excess flue gas temperature (C) 14 0, 10%, 20%, 40%, 50%, 60%, 80% 200, 300, 400, 500
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


