Question: At a particular temperature, K-7.0 x 10- for the reaction 2 CO2(9) 2 CO(g) + O2(9) If 4.0 moles of CO2 is initially placed
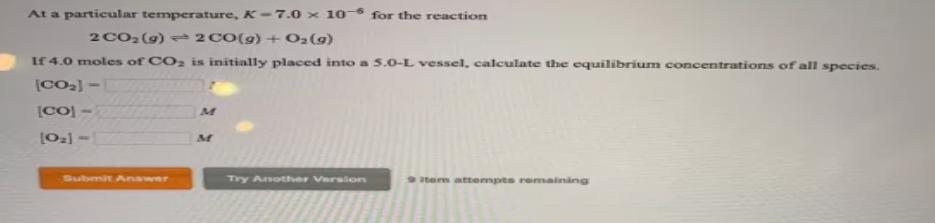
At a particular temperature, K-7.0 x 10- for the reaction 2 CO2(9) 2 CO(g) + O2(9) If 4.0 moles of CO2 is initially placed into a 5.0-L vessel, calculate the equilibrium concentrations of all species. [CO) - [021 - Submit Anawer Try Anothher Versiors tem attempts remaining
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock

Eablewin 2C0cg 2004 Ozca Givew Ozcg 4 mole of Cor in 5L Vessel i K 70x106 So conceutratio... View full answer

Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


