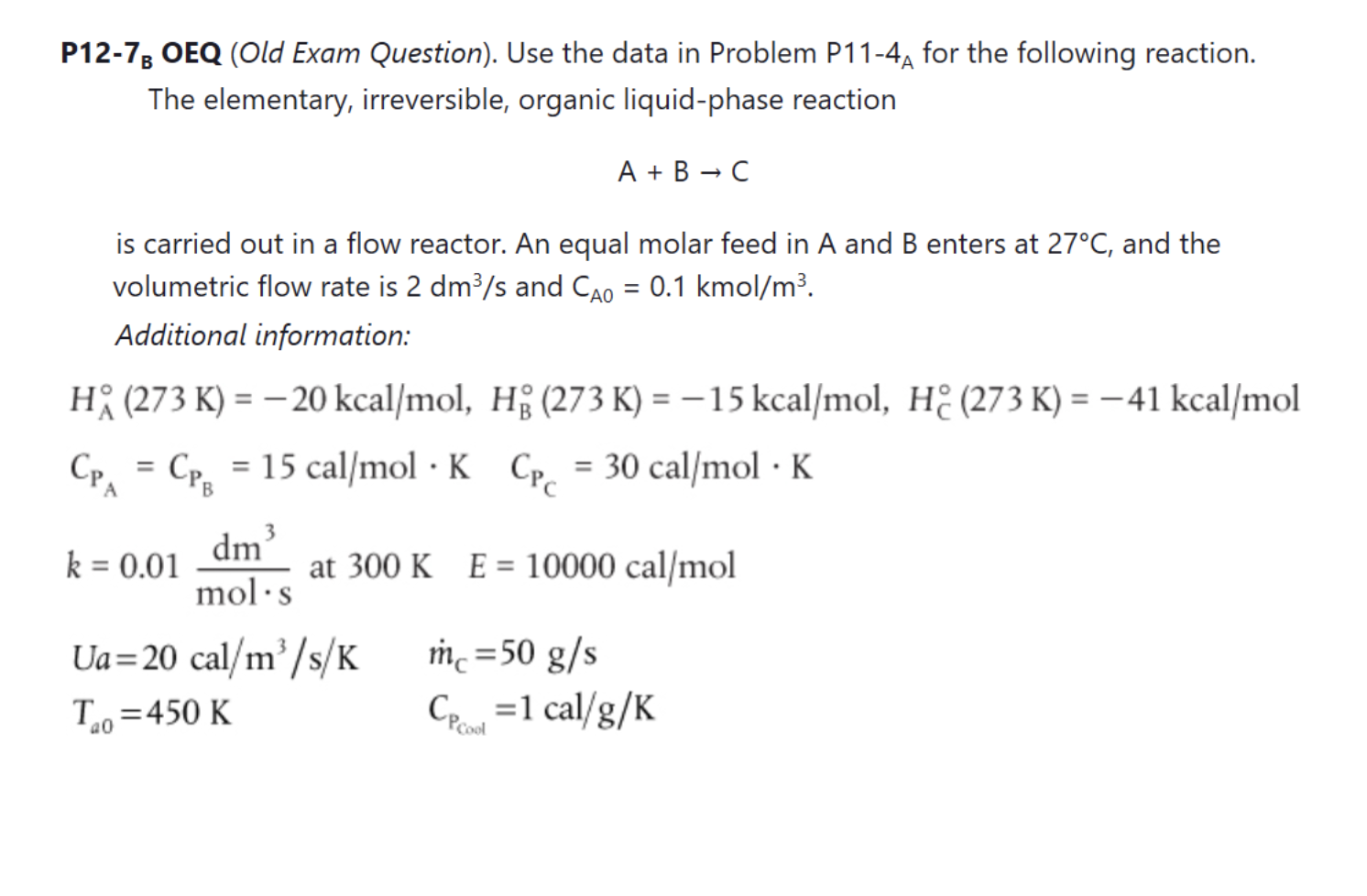
Question: Please do parts a, b, e only P12-78 OEQ (Old Exam Question). Use the data in Problem P11-4A for the following reaction. The elementary, irreversible,
Please do parts a, b, e only
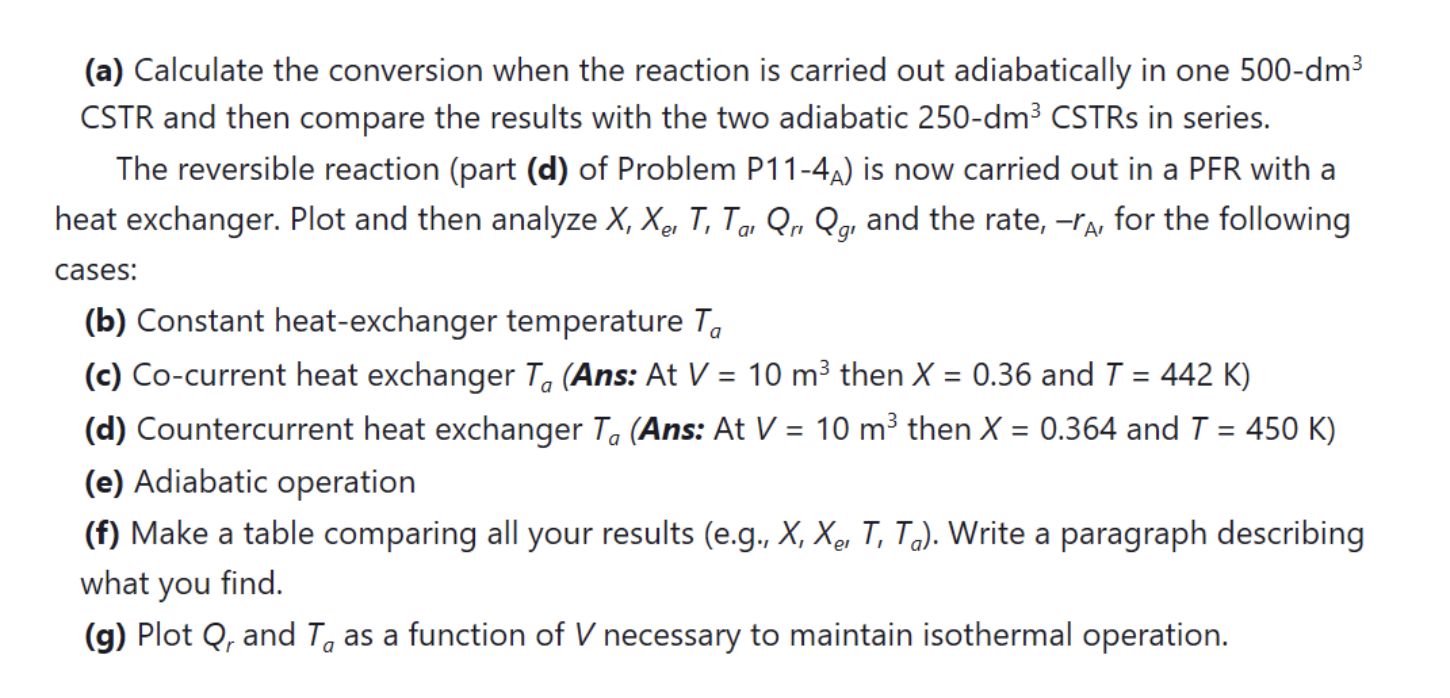
P12-78 OEQ (Old Exam Question). Use the data in Problem P11-4A for the following reaction. The elementary, irreversible, organic liquid-phase reaction A + B - C = is carried out in a flow reactor. An equal molar feed in A and B enters at 27C, and the volumetric flow rate is 2 dm3/s and Cao = 0.1 kmol/m3. Additional information: H; (273 K) = 20 kcal/mol, H(273 K) = -15 kcal/mol, H (273 K) = -41 kcal/mol Cp= Cpg = 15 cal/mol K Cpc = 30 cal/mol K . = = = A B dm3 k = 0.01 at 300 K E = 10000 cal/mol mol.s Ua=20 cal/m/s/K me = 50 g/s T=450 K Cp =1 cal/g/K a0 (a) Calculate the conversion when the reaction is carried out adiabatically in one 500-dm3 CSTR and then compare the results with the two adiabatic 250-dm3 CSTRs in series. The reversible reaction (part (d) of Problem P11-4a) is now carried out in a PFR with a heat exchanger. Plot and then analyze X, Xe, T, Tg, Qro Qg, and the rate, -ra, for the following cases: = = = = (b) Constant heat-exchanger temperature Ta (c) Co-current heat exchanger T, (Ans: At V = 10 m3 then X = 0.36 and T = 442 K) (d) Countercurrent heat exchanger Ta (Ans: At V = 10 m3 then X = 0.364 and T = 450 K) (e) Adiabatic operation (f) Make a table comparing all your results (e.g., X, Xe, T, T.). Write a paragraph describing what you find. (g) Plot Q, and T, as a function of V necessary to maintain isothermal operation. a
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


