Question: S298 1. Construct a speciation diagram for the water-sulfide system (H2Saq, HS, and S?) for 0.0001 m total sulfide at 50 C from pH 0
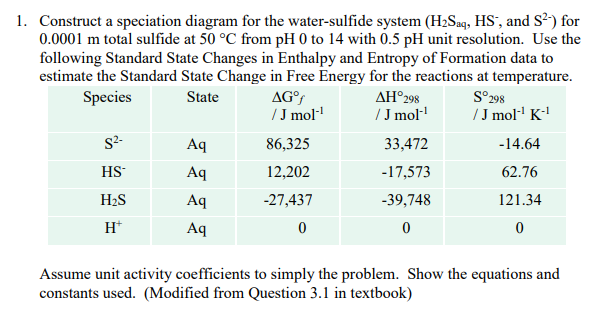
S298 1. Construct a speciation diagram for the water-sulfide system (H2Saq, HS, and S?) for 0.0001 m total sulfide at 50 C from pH 0 to 14 with 0.5 pH unit resolution. Use the following Standard State Changes in Enthalpy and Entropy of Formation data to estimate the Standard State Change in Free Energy for the reactions at temperature. Species State AG 298 /J mol /J moll /J mol-'K Aq 86,325 33,472 -14.64 HS Aq 12,202 -17,573 62.76 H2S Aq -27,437 -39,748 121.34 H Aq 0 0 0 S2 Assume unit activity coefficients to simply the problem. Show the equations and constants used. (Modified from Question 3.1 in textbook)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


