Question: sabon 12 tyet Wered Consider a solution that contains 0.50 moles of KF and as moles of HF in 1.0 L of water. If 0.10
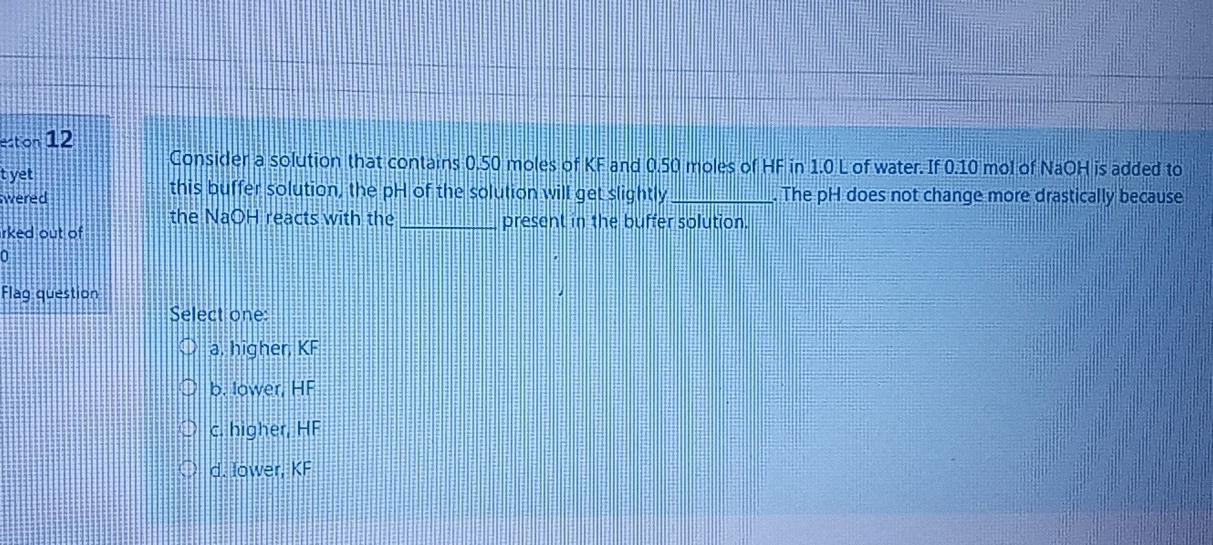
sabon 12 tyet Wered Consider a solution that contains 0.50 moles of KF and as moles of HF in 1.0 L of water. If 0.10 mol of NaOH is added to this buffer solution, the pH of the solution will get slightly The pH does not change more drastically because the Naoh readts with the present in the buffer solution. Erked out of Flag question Select one: a higher KF blower HF und higher HF d. Lower KF
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


