Question: same Now you are ready to study the reaction rate of H2O2 in solution. You place 30.0 mL of a solution containing 5.7 % H2O2
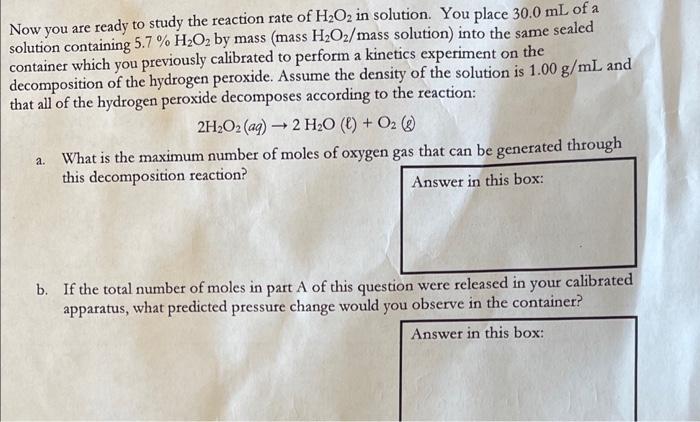
same Now you are ready to study the reaction rate of H2O2 in solution. You place 30.0 mL of a solution containing 5.7 % H2O2 by mass (mass H2O2/mass solution) into the sealed container which you previously calibrated to perform a kinetics experiment on the decomposition of the hydrogen peroxide. Assume the density of the solution is 1.00 g/mL and that all of the hydrogen peroxide decomposes according to the reaction: 2H2O2 (aq) + 2 H20 () + O2( a. What is the maximum number of moles of oxygen gas that can be generated through this decomposition reaction? Answer in this box: - b. If the total number of moles in part A of this question were released in your calibrated apparatus, what predicted pressure change would you observe in the container? Answer in this box
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


