Question: Experimental Part 1 - Preparation of 4,7-Dihydroxyflavylium Chloride (DHF) A T B ago D C Figure 1. Known equilibria of the DHF cation (D)
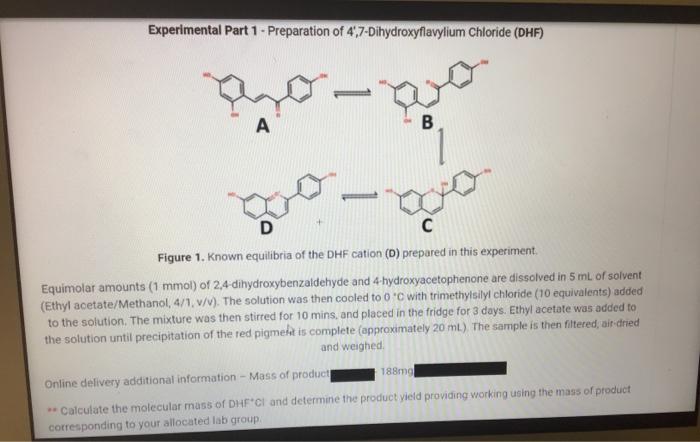
Experimental Part 1 - Preparation of 4,7-Dihydroxyflavylium Chloride (DHF) A T B ago D C Figure 1. Known equilibria of the DHF cation (D) prepared in this experiment. Equimolar amounts (1 mmol) of 2,4-dihydroxybenzaldehyde and 4-hydroxyacetophenone are dissolved in 5 mL of solvent (Ethyl acetate/Methanol, 4/1, v/v). The solution was then cooled to 0C with trimethylsilyl chloride (10 equivalents) added to the solution. The mixture was then stirred for 10 mins, and placed in the fridge for 3 days. Ethyl acetate was added to the solution until precipitation of the red pigment is complete (approximately 20 mL). The sample is then filtered, air-dried and weighed. 188mg Online delivery additional information - Mass of product **Calculate the molecular mass of DHF CI and determine the product yield providing working using the mass of product corresponding to your allocated lab group.
Step by Step Solution
3.38 Rating (160 Votes )
There are 3 Steps involved in it
Answer HO HO MW 13812 H HO MW13615 HO Dzo HO HO MW 27404 OH 1 mmol of 24dihydroxybenza... View full answer

Get step-by-step solutions from verified subject matter experts


