Question: Sample problem 4 In another analysis, a 10.00 mL sample of the cleaner was taken and placed in a 500ml volumetric flask and made up
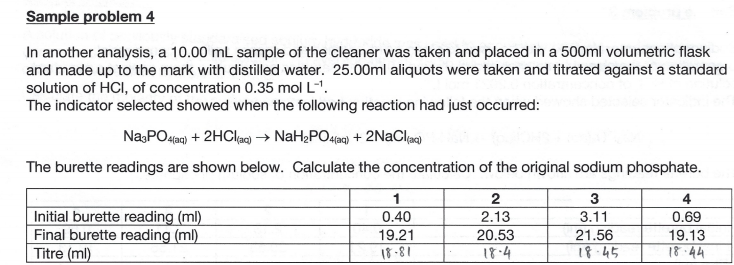
Sample problem 4 In another analysis, a 10.00 mL sample of the cleaner was taken and placed in a 500ml volumetric flask and made up to the mark with distilled water. 25.00ml aliquots were taken and titrated against a standard solution of HCl, of concentration 0.35 mol L-'. The indicator selected showed when the following reaction had just occurred: Na3PO4(aq) + 2HCl(aq) + NaH2PO4(aq) + 2NaClaa) The burette readings are shown below. Calculate the concentration of the original sodium phosphate. 1 2 3 4 Initial burette reading (ml) 0.40 2.13 3.11 0.69 Final burette reading (ml) 19.21 20.53 21.56 19.13 Titre (ml) 18-81 18.4 18.45 18.44
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


