Question: science and physic From equation Q.= = 3.00/ = 115/000 J or 115 kiloJoules Example B: The engine requires 115 kiloJoules/s = 115 kilowatts of
science and physic
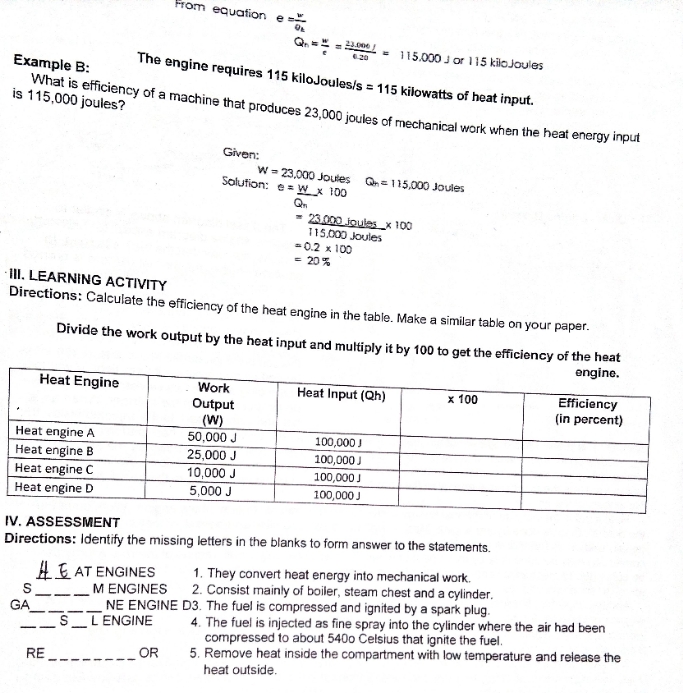
From equation Q.= = 3.00/ = 115/000 J or 115 kiloJoules Example B: The engine requires 115 kiloJoules/s = 115 kilowatts of heat input. is 115,000 joules? What is efficiency of a machine that produces 23,000 joules of mechanical work when the heat energy input Given: W = 23,000 Joutes Solution: e = W x 100 Q. = 1 15,000 Joules - 23 000 joules_x 100 115,000 Joules = 0.2 x 100 = 20% III. LEARNING ACTIVITY Directions: Calculate the efficiency of the heat engine in the table. Make a similar table on your paper. Divide the work output by the heat input and multiply it by 100 to get the efficiency of the heat engine. Heat Engine Work Heat Input (Qh) x 100 Efficiency Output (in percent) (W) Heat engine A 50,000 J 100,000] Heat engine B 25,000 J 100,000 Heat engine C 10,000 J 100,000 J Heat engine D 5,000 J 100,000 IV. ASSESSMENT Directions: Identify the missing letters in the blanks to form answer to the statements. # E AT ENGINES 1. They convert heat energy into mechanical work. S M ENGINES 2. Consist mainly of boiler, steam chest and a cylinder. GA_ NE ENGINE D3. The fuel is compressed and ignited by a spark plug. S _L ENGINE 4. The fuel is injected as fine spray into the cylinder where the air had been compressed to about 5400 Celsius that ignite the fuel. _OR 5. Remove heat inside the compartment with low temperature and release the RE_ heat outside
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


