Question: Second-order reactions in a batch reactor. In class, we have derived the following relation that expresses the consumption of compound A in a well-mixed batch
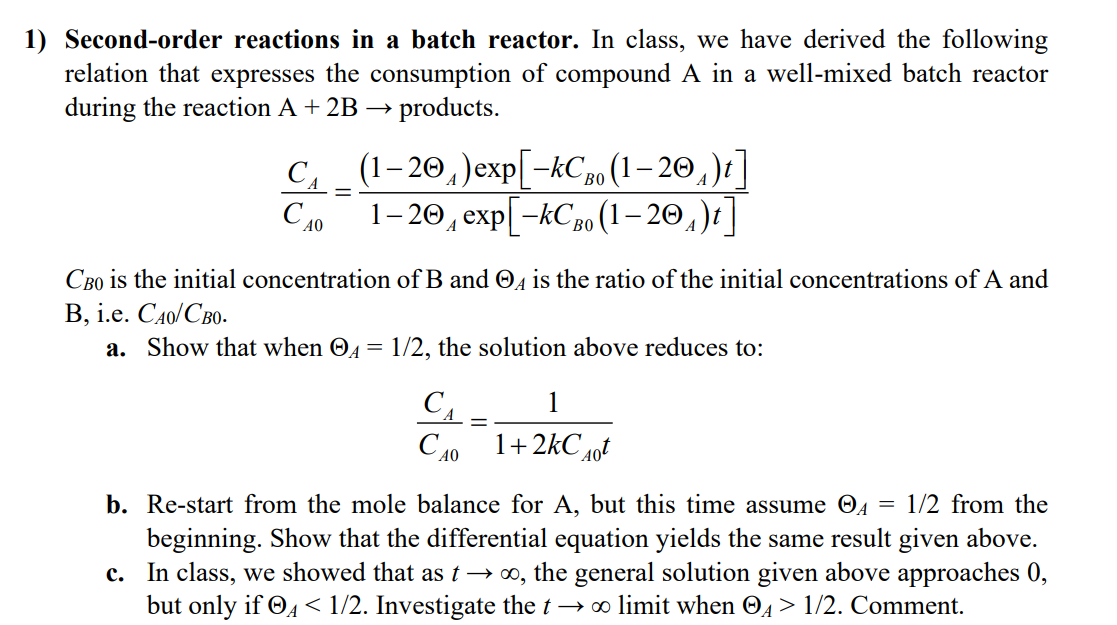
Second-order reactions in a batch reactor. In class, we have derived the following relation that expresses the consumption of compound A in a well-mixed batch reactor during the reaction A+2B products. CA0CA=12Aexp[kCB0(12A)t](12A)exp[kCB0(12A)t] CB0 is the initial concentration of B and A is the ratio of the initial concentrations of A and B, i.e. CA0/CB0. a. Show that when A=1/2, the solution above reduces to: CA0CA=1+2kCA0t1 b. Re-start from the mole balance for A, but this time assume A=1/2 from the beginning. Show that the differential equation yields the same result given above. c. In class, we showed that as t, the general solution given above approaches 0 , but only if A1/2. Comment
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


