Question: SECTION A: Answer ALL the questions in this section 1. a) The hydrogenation of carbon dioxide (CO2) to produce methanol (CH3OH) has been studied using
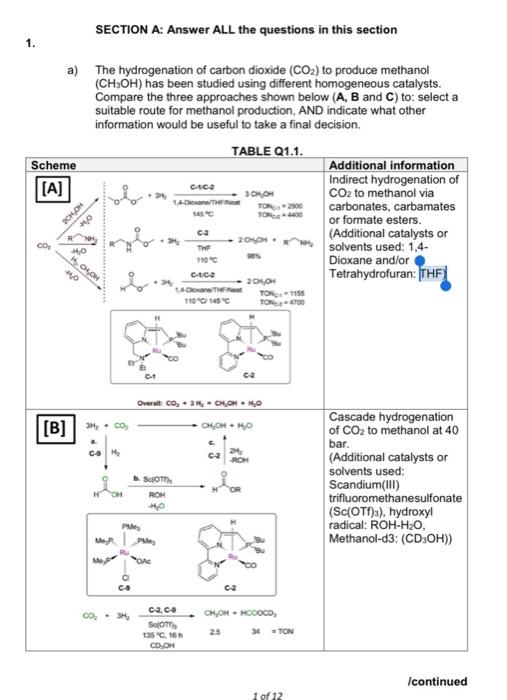
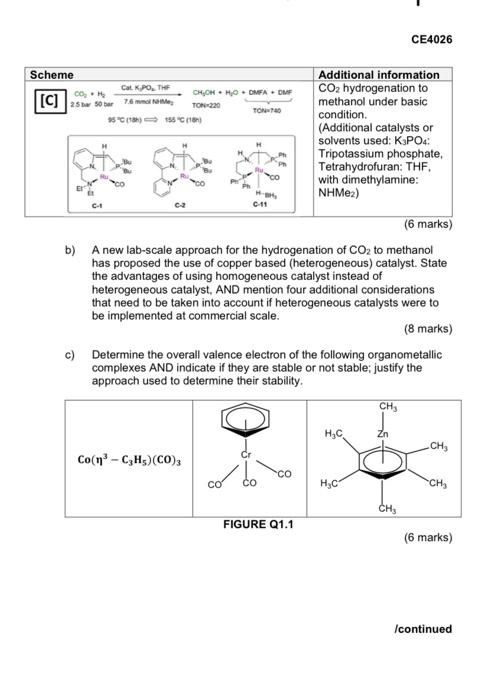
SECTION A: Answer ALL the questions in this section 1. a) The hydrogenation of carbon dioxide (CO2) to produce methanol (CH3OH) has been studied using different homogeneous catalysts. Compare the three approaches shown below (A, B and C) to: Select a suitable route for methanol production, AND indicate what other information would be useful to take a final decision. TABLE Q1.1. Scheme Additional information [A] Indirect hydrogenation of CO2 to methanol via carbonates, carbamates or formate esters. (Additional catalysts or solvents used: 1,4 Dioxane and/or Tetrahydrofuran: THE Once 1-O HOME TON 2500 #0 ca 204 CO 0 TH H.COM 0 CUC 1 / 110C 15C 24H TON1150 TON-6700 ca Overall Co, 3H, CHON MO [B] 3. CHOHHO COM 24 ROH SOTT Cascade hydrogenation of CO2 to methanol at 40 bar. (Additional catalysts or solvents used: Scandium(III) trifluoromethanesulfonate (Sc(OTf)a), hydroxyl radical: ROH-H20, Methanol-d3: (CD,OH)) ROH PA Mep Pue OAC c Co co, CH OHHCOOCD, c2.c. SOTY 135.15 COOH 34 TON Icontinued 1 of 12 CE4026 Scheme Cat KPOTHE CO, CHOHHOOMAD [C] asso 7.6 mm TON220 TON-740 *5 (18) 158 (1) Additional information CO2 hydrogenation to methanol under basic condition (Additional catalysts or solvents used: K3PO4: Tripotassium phosphate, Tetrahydrofuran: THF, with dimethylamine: NHMea) CH 0-11 (6 marks) b) A new lab-scale approach for the hydrogenation of CO2 to methanol has proposed the use of copper based (heterogeneous) catalyst. State the advantages of using homogeneous catalyst instead of heterogeneous catalyst, AND mention four additional considerations that need to be taken into account if heterogeneous catalysts were to be implemented at commercial scale. (8 marks) c) Determine the overall valence electron of the following organometallic complexes AND indicate if they are stable or not stable; justify the approach used to determine their stability CH, 4 Zn CH Co(nh - CH)(CO)3 CO CO , CH, FIGURE 21.1 (6 marks) Icontinued SECTION A: Answer ALL the questions in this section 1. a) The hydrogenation of carbon dioxide (CO2) to produce methanol (CH3OH) has been studied using different homogeneous catalysts. Compare the three approaches shown below (A, B and C) to: Select a suitable route for methanol production, AND indicate what other information would be useful to take a final decision. TABLE Q1.1. Scheme Additional information [A] Indirect hydrogenation of CO2 to methanol via carbonates, carbamates or formate esters. (Additional catalysts or solvents used: 1,4 Dioxane and/or Tetrahydrofuran: THE Once 1-O HOME TON 2500 #0 ca 204 CO 0 TH H.COM 0 CUC 1 / 110C 15C 24H TON1150 TON-6700 ca Overall Co, 3H, CHON MO [B] 3. CHOHHO COM 24 ROH SOTT Cascade hydrogenation of CO2 to methanol at 40 bar. (Additional catalysts or solvents used: Scandium(III) trifluoromethanesulfonate (Sc(OTf)a), hydroxyl radical: ROH-H20, Methanol-d3: (CD,OH)) ROH PA Mep Pue OAC c Co co, CH OHHCOOCD, c2.c. SOTY 135.15 COOH 34 TON Icontinued 1 of 12 CE4026 Scheme Cat KPOTHE CO, CHOHHOOMAD [C] asso 7.6 mm TON220 TON-740 *5 (18) 158 (1) Additional information CO2 hydrogenation to methanol under basic condition (Additional catalysts or solvents used: K3PO4: Tripotassium phosphate, Tetrahydrofuran: THF, with dimethylamine: NHMea) CH 0-11 (6 marks) b) A new lab-scale approach for the hydrogenation of CO2 to methanol has proposed the use of copper based (heterogeneous) catalyst. State the advantages of using homogeneous catalyst instead of heterogeneous catalyst, AND mention four additional considerations that need to be taken into account if heterogeneous catalysts were to be implemented at commercial scale. (8 marks) c) Determine the overall valence electron of the following organometallic complexes AND indicate if they are stable or not stable; justify the approach used to determine their stability CH, 4 Zn CH Co(nh - CH)(CO)3 CO CO , CH, FIGURE 21.1 (6 marks) Icontinued
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


