Question: Section I 1) In the miscibility experiments of three components, (i.e. iso-butanol, (C4H7OH), benzene (C6H6), and water (H2O)), at 25C and 1 bar the following
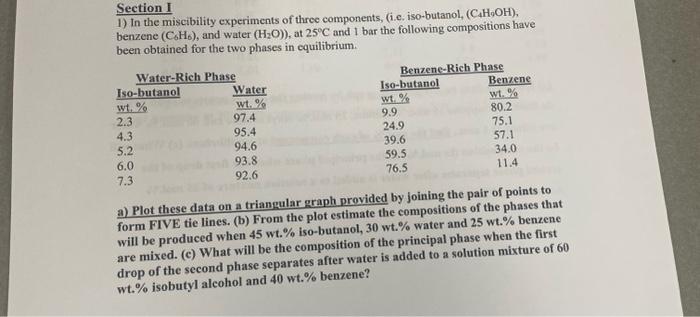
Section I 1) In the miscibility experiments of three components, (i.e. iso-butanol, (C4H7OH), benzene (C6H6), and water (H2O)), at 25C and 1 bar the following compositions have been obtained for the two phases in equilibrium. a) Plot these data on a trianqular araph provided by joining the paur ux puma. form FIVE tie lines. (b) From the plot estimate the compositions of the phases that will be produced when 45wt% iso-butanol, 30wt% water and 25wt% benzene are mixed. (c) What will be the composition of the principal phase when the first drop of the second phase separates after water is added to a solution mixture of 60 wt. % isobutyl alcohol and 40 wt. % benzene
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


