Question: See attached please Using your periodic table and the chart shown below, determine the mass defect of a lithium-7 nucleus, given that its actual atomic
See attached please
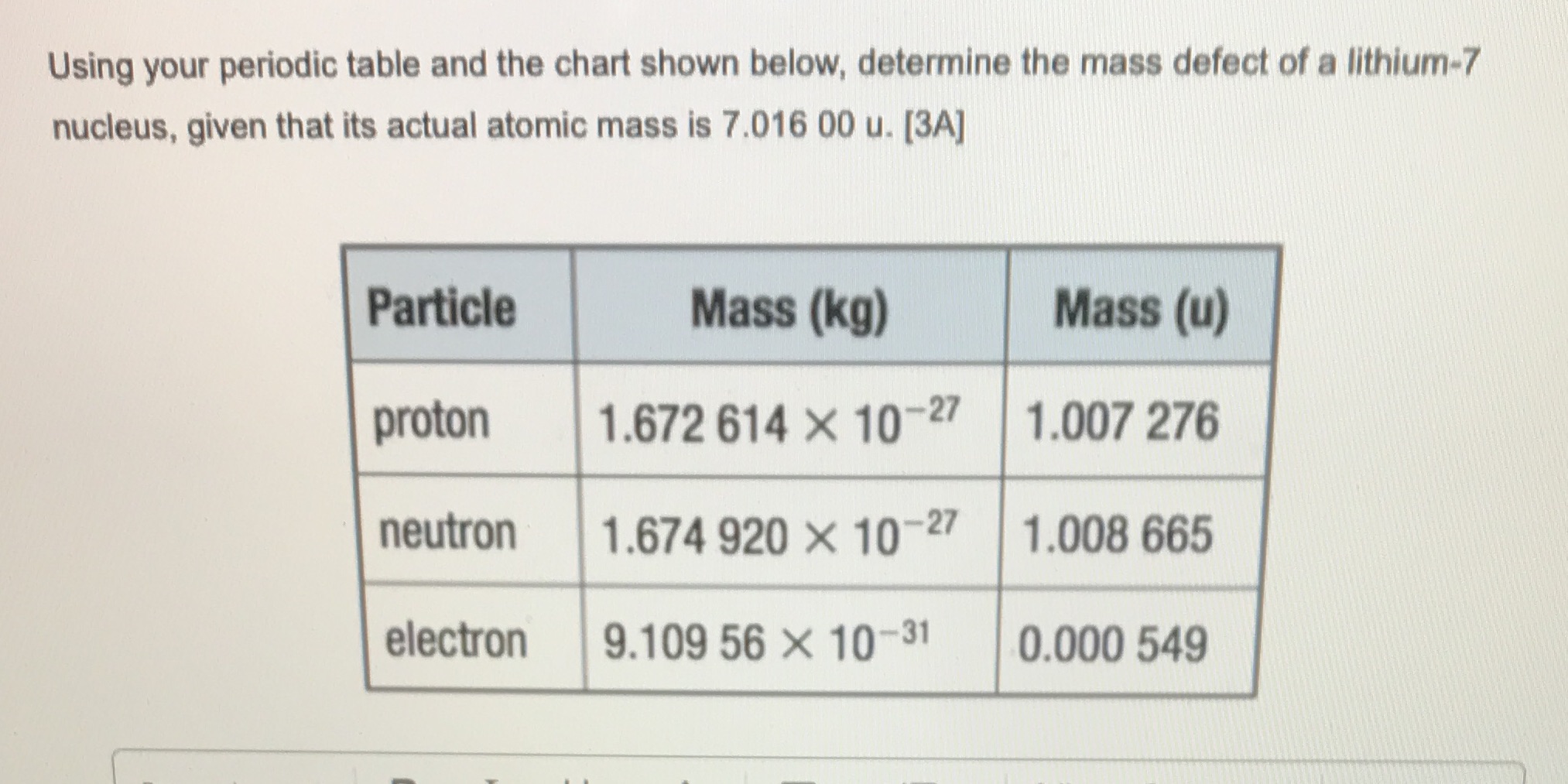
Using your periodic table and the chart shown below, determine the mass defect of a lithium-7 nucleus, given that its actual atomic mass is 7.016 00 u. [3A] Particle Mass (kg) Mass (u) proton 1.672 614 X 10-27 1.007 276 neutron 1.674 920 X 10-27 1.008 665 electron 9.109 56 X 10-31 0.000 549
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


