Question: Select all that apply Which statements correctly interpret the boiling point graph shewn? 5 elect alf that apply. For a series of binary hydrides in
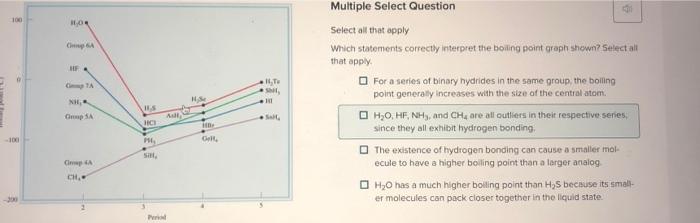
Select all that apply Which statements correctly interpret the boiling point graph shewn? 5 elect alf that apply. For a series of binary hydrides in the sarme group, the boiling point generally increases with the size of the central atom. H2O,HF3NH3, and CH4 are all cutliers in their respective. series; since they all exhibit hydrofen bonding. The existence of hydrogen bonding can cause a smailer tholecule to have a higher boiling point than a larger analog H2O has a much higher boiling point than H2S because its smalkef molecules can pack closer to gether in the liquid state
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


