Question: Select the correct electron-dot formulas. Draw the electron-dot formula for the element sulfur. Select the correct electron-dot formulas. You can refer to the periodic table
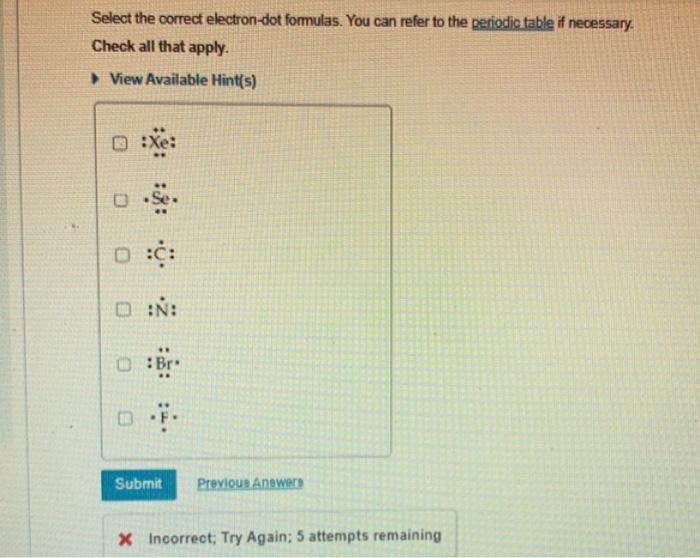
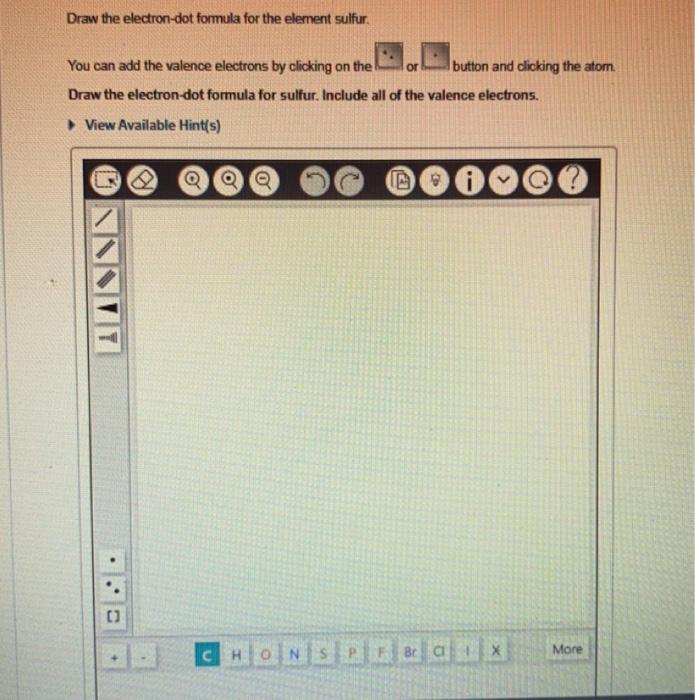

Select the correct electron-dot formulas. You can refer to the periodic table if necessary. Check all that apply. View Available Hint(s) :Xe: - Se :CC :N: :BB * F. x Incorrect; Try Again: 5 attempts remaining Draw the electron-dot formula for the element sulfur. You can add the valence electrons by clicking on the or button and clicking the atom. Draw the electron-dot formula for sulfur. Include all of the valence electrons. View Available Hint(s) An electron-dot formula, or Lewis electron-dot structure, can be used to represent the number of valence electrons in the atom of an element. The symbol of an element is used to represent the core of the atom, and the valence electrons are denoted using dots arranged around the symbol. The maximum number of electron dots that an element can have is eight. The electrons are added one at a time moving around the core atom until each side of the atom has a single dot before the electrons are paired. The first four electrons are arranged singly. Pairing of electrons occurs from the fifth electron. For example, the electron-dot formula for chlorine, Cl, which has seven valence electrons, can be represented as follows: Four electrons are arranged singly first around the element's symbol. The fifth, sixth, and seventh electrons pair up with first three electrons. The fourth electron is unpaired
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


