Question: Select the correct response in the drop down box in the following 5 sentences. a. The strongest bond in which electrons are shared will form
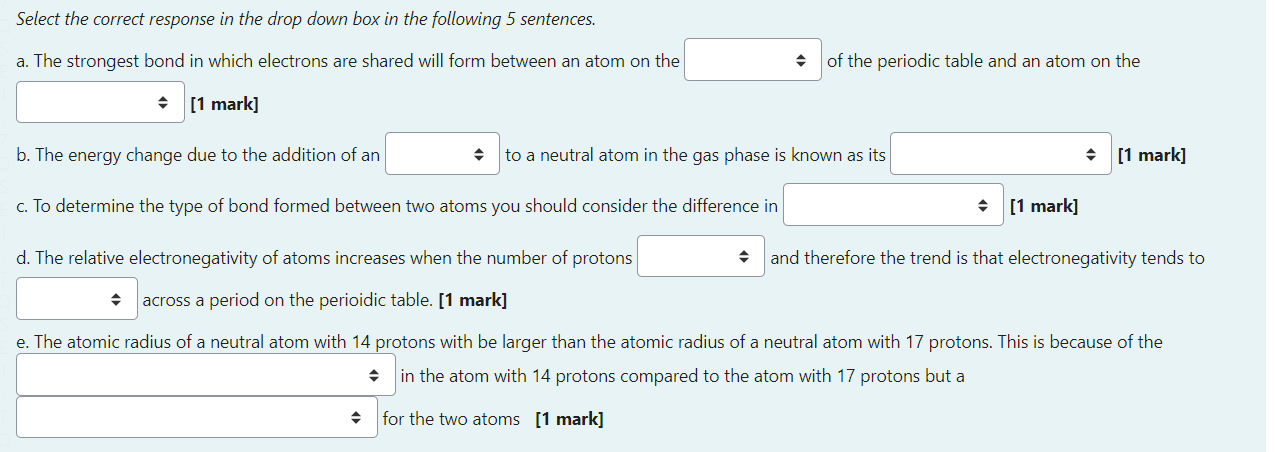
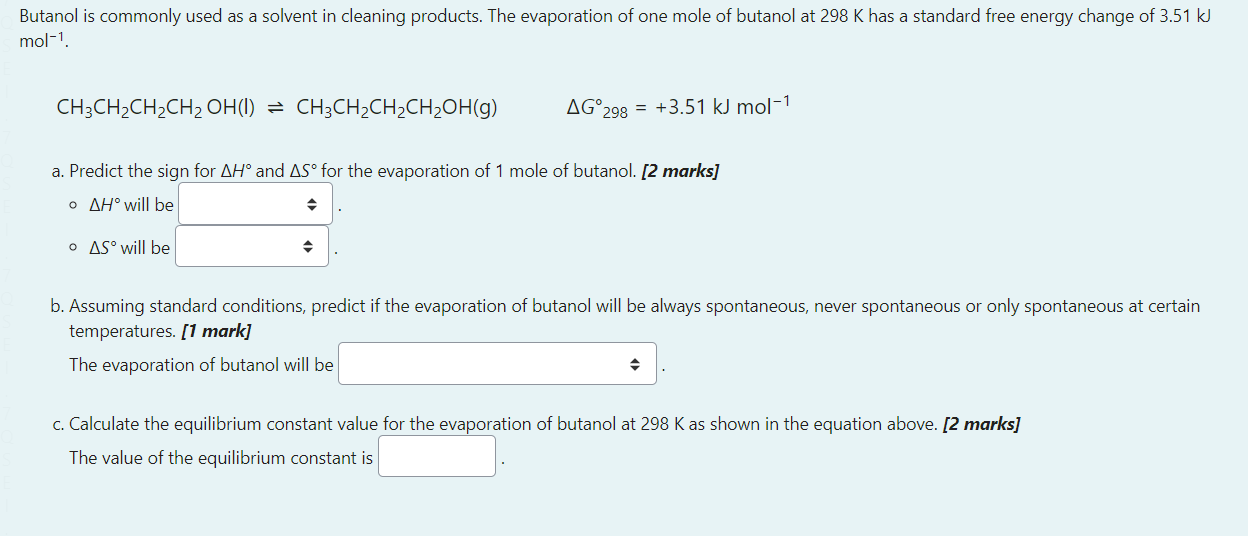
Select the correct response in the drop down box in the following 5 sentences. a. The strongest bond in which electrons are shared will form between an atom on the of the periodic table and an atom on the [1 mark] b. The energy change due to the addition of an to a neutral atom in the gas phase is known as its [1 mark] c. To determine the type of bond formed between two atoms you should consider the difference in [1 mark] d. The relative electronegativity of atoms increases when the number of protons and therefore the trend is that electronegativity tends to across a period on the perioidic table. [1 mark] e. The atomic radius of a neutral atom with 14 protons with be larger than the atomic radius of a neutral atom with 17 protons. This is because of the in the atom with 14 protons compared to the atom with 17 protons but a for the two atoms [1 mark] Butanol is commonly used as a solvent in cleaning products. The evaporation of one mole of butanol at 298K has a standard free energy change of 3.51kJ mol1. CH3CH2CH2CH2OH(l)CH3CH2CH2CH2OH(g)G298=+3.51kJmol1 a. Predict the sign for H and S for the evaporation of 1 mole of butanol. [2 marks] - H will be - S will be b. Assuming standard conditions, predict if the evaporation of butanol will be always spontaneous, never spontaneous or only spontaneous at certain temperatures. [1 mark] The evaporation of butanol will be c. Calculate the equilibrium constant value for the evaporation of butanol at 298K as shown in the equation above. [2 marks] The value of the equilibrium constant is
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


