Question: selective. Offer some rationalization for the creation of new stereogenic centres in the first and second reactions. 4. What is happening in stereochemical terms in
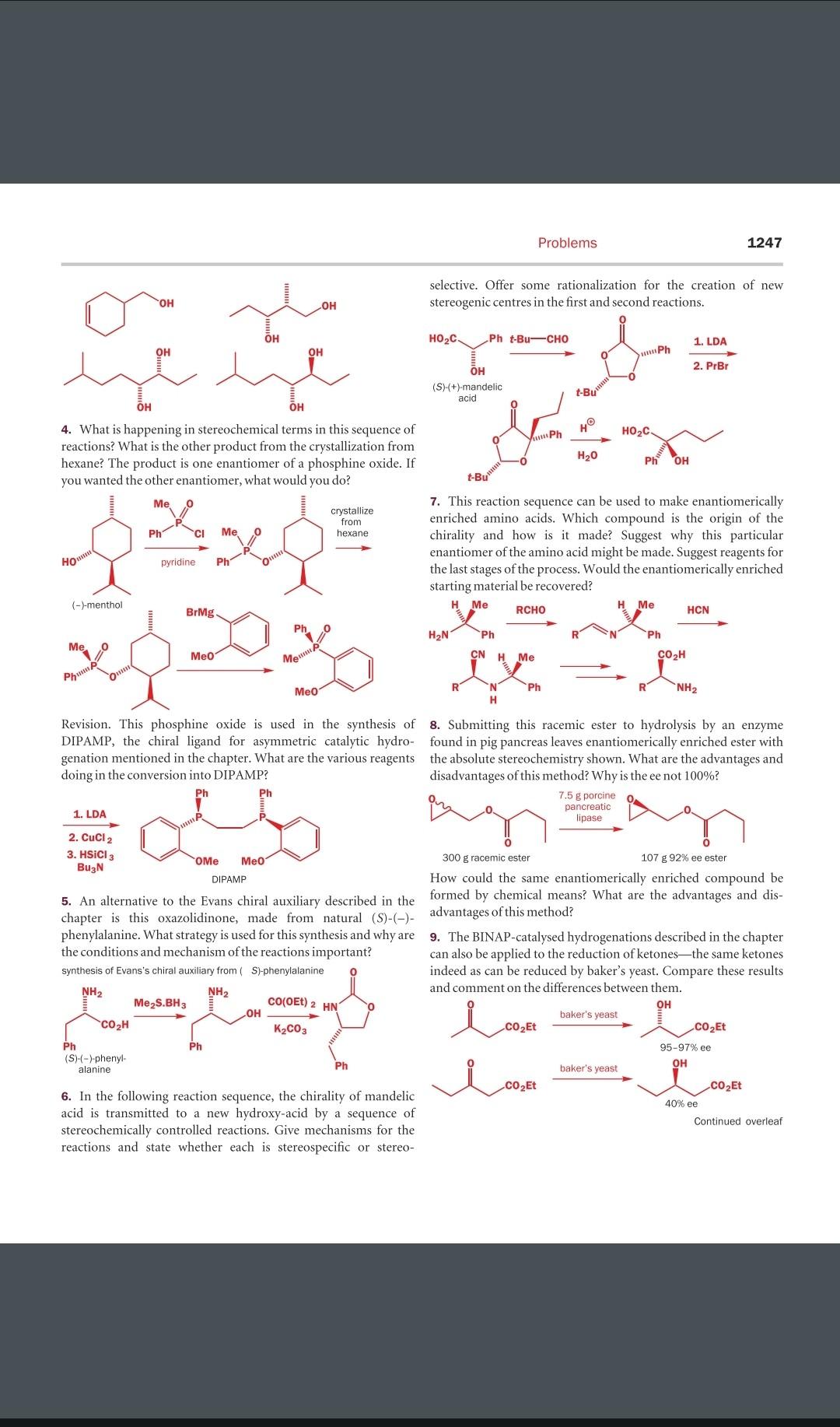
selective. Offer some rationalization for the creation of new stereogenic centres in the first and second reactions. 4. What is happening in stereochemical terms in this sequence of reactions? What is the other product from the crystallization from hexane? The product is one enantiomer of a phosphine oxide. If you wanted the other enantiomer, what would you do? 7. This reaction sequence can be used to make enantiomerically enriched amino acids. Which compound is the origin of the chirality and how is it made? Suggest why this particular enantiomer of the amino acid might be made. Suggest reagents for the last stages of the process. Would the enantiomerically enriched starting material be recovered? Revision. This phosphine oxide is used in the synthesis of 8. Submitting this racemic ester to hydrolysis by an enzyme DIPAMP, the chiral ligand for asymmetric catalytic hydro- found in pig pancreas leaves enantiomerically enriched ester with genation mentioned in the chapter. What are the various reagents the absolute stereochemistry shown. What are the advantages and doing in the conversion into DIPAMP? disadvantages of this method? Why is the ee not 100% ? How could the same enantiomerically enriched compound be 5. An alternative to the Evans chiral auxiliary described in the formed by chemical means? What are the advantages and dischapter is this oxazolidinone, made from natural (S)() - advantages of this method? phenylalanine. What strategy is used for this synthesis and why are 9. The BINAP-catalysed hydrogenations described in the chapter the conditions and mechanism of the reactions important? can also be applied to the reduction of ketones-the same ketones indeed as can be reduced by baker's yeast. Compare these results and comment on the differences between them. 6. In the following reaction sequence, the chirality of mandelic acid is transmitted to a new hydroxy-acid by a sequence of stereochemically controlled reactions. Give mechanisms for the reactions and state whether each is stereospecific or stereo- selective. Offer some rationalization for the creation of new stereogenic centres in the first and second reactions. 4. What is happening in stereochemical terms in this sequence of reactions? What is the other product from the crystallization from hexane? The product is one enantiomer of a phosphine oxide. If you wanted the other enantiomer, what would you do? 7. This reaction sequence can be used to make enantiomerically enriched amino acids. Which compound is the origin of the chirality and how is it made? Suggest why this particular enantiomer of the amino acid might be made. Suggest reagents for the last stages of the process. Would the enantiomerically enriched starting material be recovered? Revision. This phosphine oxide is used in the synthesis of 8. Submitting this racemic ester to hydrolysis by an enzyme DIPAMP, the chiral ligand for asymmetric catalytic hydro- found in pig pancreas leaves enantiomerically enriched ester with genation mentioned in the chapter. What are the various reagents the absolute stereochemistry shown. What are the advantages and doing in the conversion into DIPAMP? disadvantages of this method? Why is the ee not 100% ? How could the same enantiomerically enriched compound be 5. An alternative to the Evans chiral auxiliary described in the formed by chemical means? What are the advantages and dischapter is this oxazolidinone, made from natural (S)() - advantages of this method? phenylalanine. What strategy is used for this synthesis and why are 9. The BINAP-catalysed hydrogenations described in the chapter the conditions and mechanism of the reactions important? can also be applied to the reduction of ketones-the same ketones indeed as can be reduced by baker's yeast. Compare these results and comment on the differences between them. 6. In the following reaction sequence, the chirality of mandelic acid is transmitted to a new hydroxy-acid by a sequence of stereochemically controlled reactions. Give mechanisms for the reactions and state whether each is stereospecific or stereo
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


