Question: Self Assessment 15.3 Consider the given acid ionization constants. Identify the strongest conjugate base. Acid K HF (aq) 3.5x10-4 HC7H5O2 (aq) 6.5x10-5 |HClO,(aq) 1.1x10-2
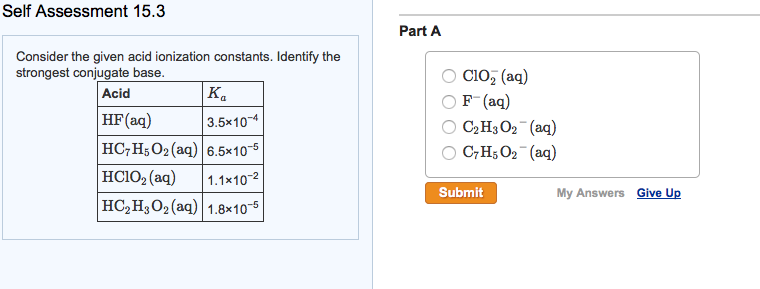
Self Assessment 15.3 Consider the given acid ionization constants. Identify the strongest conjugate base. Acid K HF (aq) 3.5x10-4 HC7H5O2 (aq) 6.5x10-5 |HClO,(aq) 1.1x10-2 HCH3O (aq) 1.8x10-5 Part A ClO (aq) F- (aq) CH3O2 (aq) C7H5O2 (aq) Submit My Answers Give Up
Step by Step Solution
3.35 Rating (155 Votes )
There are 3 Steps involved in it
Strongest acid is the acid having lower pK values Wr... View full answer

Get step-by-step solutions from verified subject matter experts


